PFD Management Shares Update PFD/iOI’s FIH Implant
PFD MANAGEMENT
OPPORTUNITY FUND 3001, LLC
23161 Lake Center Drive, Suite 100
Lake Forest, CA 92630
Telephone (661) 665-6074, Toll free (888) 475-4748
FOR IMMEDIATE RELEASE
DATELINE: June 15, 2023 Lake Forest California
Announced today by Robert Pryke PFD Capital Partners, LLC, Director, and Fund manager for PFD Management Opportunity Fund 3001, LLC, additional points of interest related to its PFD/iOrthopedics portfolio project.
Final Hurdle Reached For FIH Implant of the iKnee
PFD Capital Partners, LLC is releasing the following information in relation to the progress of one of the medical technology companies within the PFD Management Opportunity Fund 3001, LLC. PFD/iOrthopedics, Inc. (PFD/iOI) has been in the development of an innovative solution for individuals with severe debilitating joint issues related to injury or Osteoarthritis (OA).
As has been discussed in previous releases, PFD/iOI is the developer of several novel, patented medical devices that are all focused on bringing renewed function to patients suffering from a variety of orthopedic issues. Specifically, over the past two years, the company has focused on the development of its Resilient Arthroplasty Device (RAD) technology and its first product candidate using the RAD technology; the iKnee.
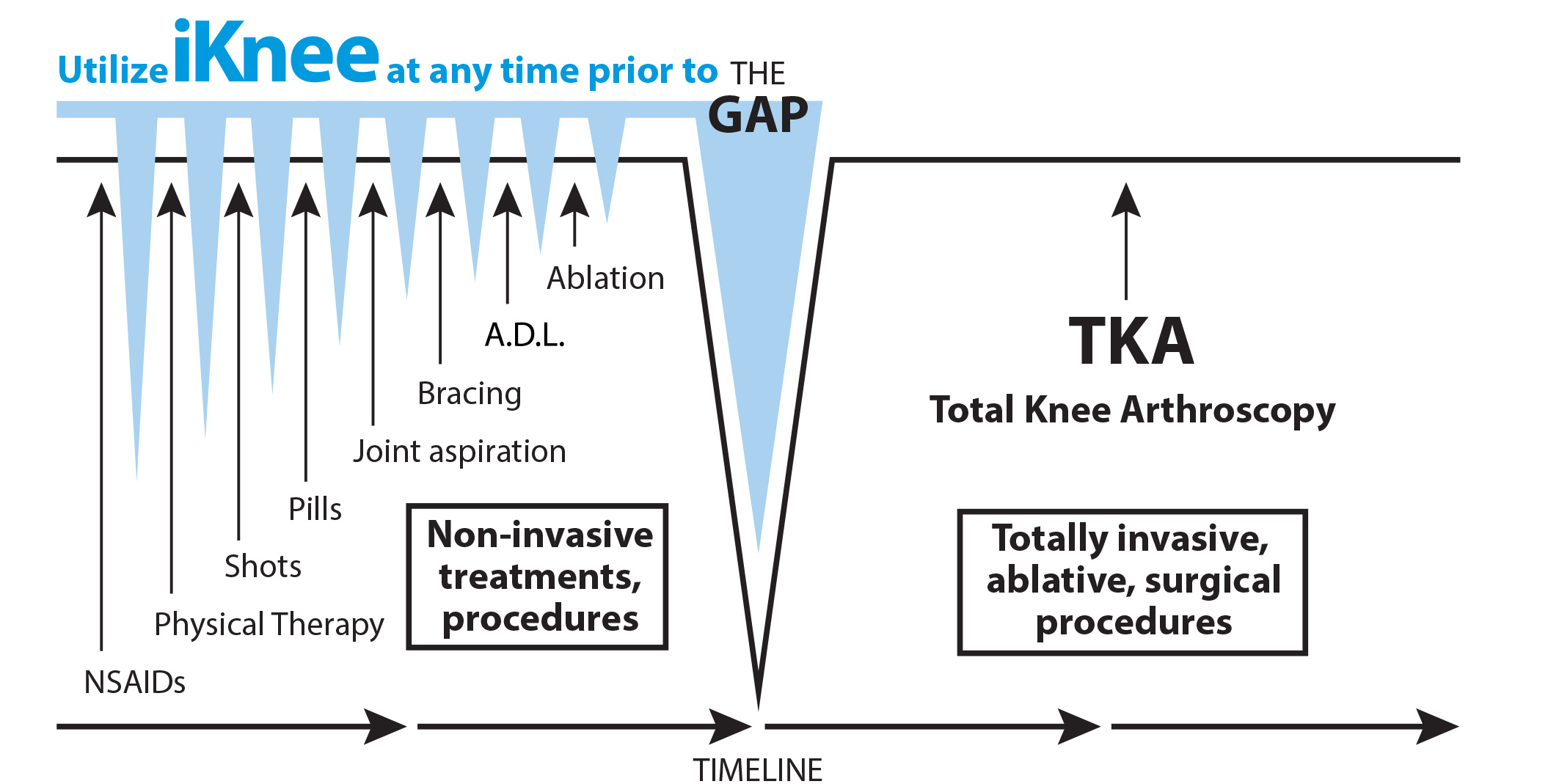
The graphic below shows how the iKnee will be able to fill the gap between minimally effective conservative treatment and the radically invasive Total Knee Arthroplasty (TKA).
Today, PFD/iOI announced that the final barriers to moving forward with its First-In-Human (FIH) implant of the iKnee have been met. As a result, PFD/iOI is hoping to schedule the FIH implant before the end of Q3, 2023.
The iKnee was created to fill the gap between the costly, inefficient, and non-therapeutic conservative care approaches and the invasive metal joint replacements of a Total Knee Arthroplasty (TKA) that limit care options to deal with knee pain and reduce daily activities and quality of life for patients suffering from osteoarthritis. By creating an arthroscopically implantable device that does not require the wide open joint resection in a patient, with the removal of large sections of bone, and the implantation of heavy hardware made from metals and hard polyethylene prosthetics, the RAD/iKnee allows a patient to be literally back on their feet in less time, at a lower cost, and with less chance of complications as those of TKA’s.

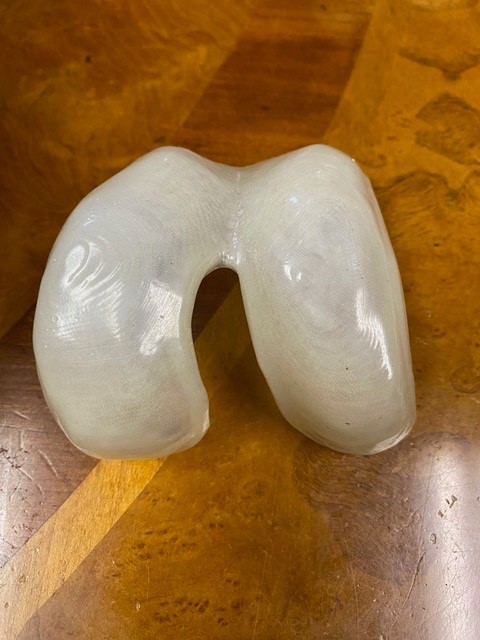
The images here show the effects of vapor smoothing of the iKnee implant after its initial 3D printing. This process provides the optimal surface for providing durability and comfort as the cushion for the distal femur’s movement in the knee joint.
iKnee Comfort Study
The RAD/iKnee technology has received an Investigational Device Exemption (IDE) from the FDA which permits a clinical trial to be done to gather data on the technology’s efficacy and safety based on its qualification of being a Non-Significant Risk (NSR) device that can provide patients who seek to avoid TKA after failed conservative management, a temporary solution to restore movement, decrease pain, and increase quality-of-life. As such, the PFD/iOI Team has created an IRB-authorized iKnee Comfort Study to examine the efficacy of the RAD/iKnee to provide its outlined benefits in an initial clinical trial of 10,000 qualified patients. In preparation for its FIH implant, PFD/iOI has been working diligently to clear the final procedural certification requirements and prototype design refinements scheduled to be completed by the end of Q3, 2023.
As part of this process, PFD/iOI has finalized its proprietary vapor-smoothing process with AM Technologies using the PURE solution. The procedure creates a perfectly smooth and durable final finish to the exterior portion of the iKnee implant that will be the main surface riding against the tibia and patellar joint. In a recent video, Dr. Christopher Woodson, who will be performing the FIH implant commented that he was very pleased with the result and is “…ready to go.” with this design.
The only remaining hurdle is the final results from the Cytotoxicity Testing from PFD/iOI’s two independent laboratories to confirm the safety of the material’s final formula and manufacture for full bio-compatibility in a human. The results are anticipated to be finished and reviewed within the next two weeks after this press release date.
The FIH implant of the iKnee will be conducted at the Memorial Care Surgical Center in Long Beach, CA. later this year. Eric Hansen, who is PFD’s Director, Co-Founder, and Clinical/Asc Operations Manager, has been selected as Patient One in the study’s cohort group.


Left: Dr. Woodson reviews the procedure for the First-In-Human Proof-Of-Concept implant with Eric Hansen, Patient One in the Comfort study to be performed at the Memorial Care Outpatient Surgical Center in Long Beach, CA pictured on the right.
Eric has suffered from debilitating knee pain for more than eight years due to injuries sustained from both sports and heavy use issues and is currently at the point where a TKA would be the traditional next step.
As such Eric meets all the criteria established by the Institutional Review Board (IRB) for the iKnee Comfort Study’s protocols as a candidate for the iKnee implant. The surgery will be performed by PFD/iOI’s Chief Investigator, Dr. Christopher Woodson, who is a board-certified orthopedic surgeon and sports medicine physician, and founder of Beach Orthopedic Specialty Institute.
Dr. Woodson will also be serving as the Principle Investigator for the iKnee Comfort Study’s ongoing trials.
Objective
The objective of the iKnee Comfort Study is to determine the iKnee’s impact on quality of life issues and its ability to allow normal activities of daily living in patients awaiting TKA. The data will be used to improve possible designs to enhance ideal patient comfort.
Study Criteria
The PFD/iOI Comfort Study is designed to allow the company to gather critical data in three major areas:
- The ability of the patient to tolerate the iKnee implant both from a bio-compatibility standpoint and an ortho-comfort level.
- The ability of the iKnee implant to decrease or eliminate the patient’s pain level as it applies to the use of the impacted joint.
- The ability of the iKnee to maintain or expand the patient’s quality of life as it pertains to daily activity, leisure and recreational movement, and/or active sports engagement.
In addition, the study will be looking at additional areas of impact including the iKnee’s ability to provide:
- better patient outcomes
- faster recovery
- lower cost
- lower risk
Candidate Selection
Candidate selection for the study will be done by PFD/iOI’s IRB which is being sponsored and managed by Advarra, who is a global leader in supplying IRB allowances, institutional bio-safety committee and research compliance services to clinical trial sponsors, CROs, hospital systems, academic medical centers, and investigators. Candidates for the trial must meet the following criteria:
Debilitating knee pain and disability after failed conservative treatment.
Be a candidate for TKA but show a reasonable expectation of temporary comfort with an iKnee implant.
- Have a BMI of 30 or less.
- Have had continual and chronic adverse issues with their knee joint.
- Have a pre-operative Visual Analogue Pain Scale (VAS) score of ≥4.
- Be referred to the study by their Primary Care Physician (PCP) or by a qualified referring orthopedic specialist.
- Have no disqualifying health conditions or risks.
Once prospective candidates have submitted their applications, they are vetted by the PFD/iOI IRB for potential inclusion into the study. The application screening process consists of a series of steps to ensure that the candidate will be a viable cohort for the program and that there is an anticipated benefit to the patient’s knee condition and quality of life as a result of participation. The application review consists of an initial patient consult with a PFD/iOI-certified physician that includes:
- Initial patient background history (HPI, PMH, FH, ROS).
- Physical examination including a VAS and joint mobility assessment.
- Initial consult on study overview, implantation procedure, and benefit/risk assessment.
- Correlation of assimilated data and ancillary lab/imaging leading to predicted beneficial iKnee outcome.
Based on this evaluation, a request for medical records, copies of the latest radiological films, which can include X-rays, CT and MRI scans, and any needed lab work is made. Once procured, an in-depth review of the applicant’s records, films, and labs is made by the qualified PFD/iOI surgeon along with the IRB.
Candidate Acceptance and Implant
If at this point the applicant is deemed to be a viable cohort, a second consult is scheduled during which the study protocols and the implant procedure are once again thoroughly reviewed. If the applicant decides to move forward, a detailed Informed Consent document is signed along with any related additional release and medical information paperwork. Once processed the patient is given a case ID and a surgery date is scheduled.
The iKnee implant procedure itself is done on an outpatient basis at a qualified PFD/iOI-authorized surgery center. The surgery takes an hour to perform and barring any complications, the patient is released and able to bear weight on the implanted knee that day.
Patient follow-up for the study is performed at 10 days, week six, week 12, week 24, and at one year plus annually thereafter, in order to assess the patient’s response to the iKnee care. In these post-operative follow-ups, the patient is assessed for:
- Comfort and level of pain
- Joint mobility
- Infection or inflammation
- Evaluation of post-op imaging
- Quality of life changes.
Post-Operative Follow-Up
The data is carefully recorded at each step and becomes part of the ongoing evaluation to ascertain several criteria including:
- iKnee design for possible improvements.
- Material integrity and bio-compatibility.
- The ability of the implant to lower or eliminate joint pain.
If, as expected, the initial FIH procedure is successful as defined by the study’s criteria of comfort, reduced pain, and bio-acceptance, enrollment of the next group of cohorts will begin immediately and evaluation and approval of other surgical centers across the US will proceed.
PFD/iOI is very proud that it has been able to bring this much-needed vision for a cure to the point of FIH use and is excited to see the results of its efforts in not only moving the product technology forward but in restoring hope and vitality to its first recipient.

Click to watch this video of Dr. Woodson examining the final iKnee prototype after vapor smoothing.

An operational Class A member and manager to the PFD Family Private Equity programs.

PFD Management, LLC is a vendor to the PFD family of private funds, specializing in sourcing, diligence, underwriting, auditing, packaging, servicing, billing/collecting, and portfolio administration of account receivables.

A medical account receivables and medical technology finance fund offering a fixed rate return with additional equity participation in targeted new medical technology opportunities.
SAFE HARBOR DECLARATION
THE STATEMENTS, PROJECTIONS AND ESTIMATES OF FUTURE PERFORMANCE OF THE COMPANY OR VARIOUS ELEMENTS OF THE COMPANY’S BUSINESS CONTAINED IN THIS MEMORANDUM THAT ARE NOT HISTORICAL FACTS ARE FORWARD-LOOKING STATEMENTS. INVESTORS SHOULD EXPECT THAT ANTICIPATED EVENTS AND CIRCUMSTANCES MAY NOT OCCUR, THAT UNANTICIPATED EVENTS AND CIRCUMSTANCES WILL OCCUR, AND THAT ACTUAL RESULTS WILL LIKELY VARY FROM THE FORWARD-LOOKING STATEMENTS. INVESTORS SHOULD BE AWARE THAT A NUMBER OF FACTORS COULD CAUSE THE FORWARD-LOOKING STATEMENTS OR PROJEC-TIONS CONTAINED IN THIS MEMORANDUM OR OTHERWISE MADE BY OR ON BEHALF OF THE COMPANY TO BE INCORRECT OR TO DIFFER MATERIALLY FROM ACTUAL RESULTS. SUCH FACTORS MAY INCLUDE, WITHOUT LIMITATION, (i) THE ABILITY OF THE COMPANY TO COMPLETE THE DEVELOPMENT OF ITS PRODUCTS IN A TIMELY MANNER, (ii) THE DEMAND FOR AND TIMING OF DEMAND FOR SUCH PRODUCTS, (iii) COMPETITION FROM OTHER PRODUCTS AND COMPANIES, (iv) THE RESULTS OF THE COMPANY’S SAFETY AND EFFICACY STUDIES, (v) THE RESULTS OF THE REGULATORY APPROVAL PROCESS, (vi) THE COMPANY’S SALES AND MAR-KETING CAPABILITIES, (vii) THE COMPANY’S ABILITY TO SELL ITS PRODUCTS PROFITABLY, (viii) THE ABILITY OF THE COMPANY’S THIRD-PARTY SUPPLIERS TO PROVIDE PRODUCTS AND SERVICES IN A RELIABLE MANNER; (ix) AVAILABILITY OF ADEQUATE DEBT AND EQUITY FINANCING, AND (x) GENERAL BUSINESS AND ECONOMIC CONDITIONS. THESE IMPORTANT FACTORS AND CERTAIN OTHER FACTORS THAT MIGHT AFFECT THE COMPA-NY’S FINANCIAL AND BUSINESS RESULTS ARE DISCUSSED IN THIS MEMORANDUM UNDER “RISK FACTORS.” THERE CAN BE NO ASSURANCE THAT THE COMPANY WILL BE ABLE TO ANTICIPATE, RESPOND TO OR ADAPT TO CHANGES IN ANY FACTORS AFFECTING THE COMPANY’S BUSINESS AND FINANCIAL RESULTS.

To learn more about the PFDMOF3001
CLICK HERE

To learn more about PFD/iOI
CLICK HERE
CONTACT
PFD Capital Partners
23161 Lake Center Dr. Ste. 100
Lake Forest, CA 92630
Email:
Office Phone:
(888) 475-4748
Fax Number:
(888) 475-4748

