NKC Announces Upcoming Animal Study for Clinical Validation of its Huygens™ – Proteus™ Robotic Arm Surgical Platform
PFD MANAGEMENT
OPPORTUNITY FUND 3001, LLC
23161 Lake Center Drive, Suite 100
Lake Forest, CA 92630
Telephone (661) 665-6074, Toll free (888) 475-4748
FOR IMMEDIATE RELEASE
DATELINE: August 30, 2022 Lake Forest California
Neuro-Kinesis Corp. (NKC) regarding the announcement of its animal studies which are to be conducted at the Technion Institute in Haifa, Israel. This announcement signals a significant move forward for the company as it seeks regulatory approval for the commercialization of its Huygens™ – Proteus™ Robotic Arm Surgical Platform.
NKC is one of the medical technologies targeted by PFD Capital Partners, INC for its PFDMOF3001 Opportunity Fund, LLC (PFDMOF3001).
PFD Capital Partners, LLC Director and Fund Manager for the PFD Management Opportunity Fund 3001, LLC, Robert Pryke announced seven points of interest relating to its portfolio project Neuro-Kinesis, Corp.
NKC Announces Upcoming Animal Study for Clinical Validation of
its Huygens™ – Proteus™ Robotic Arm Surgical Platform
NewsMulti-center trials to commence Q1 2023 at Technion and Sandia
Neuro-Kinesis Corp. (NKC) has submitted an Application for Institutional Animal Care and Use Committee Approval (IACUC) for its upcoming animal studies for validation of the Huygens™ – Proteus™ Robotic Arm Surgical Platform. An agreement has been signed with the Technion Institute in Israel to begin the animal study validation of NKC’s surgical platform in Q1 2023. The platform includes the Huygens™ Catheter, the Lorentz™ Active Sheath, and the Proteus™ Robotic Arm which have been in development over the last several years.

Dr. Eli Gang
The study will be headed by NKC Chief Electrophysiology Officer, Dr. Eli S.Gang, MD (Clinical Professor of Medicine, Geffen School of Medicine at UCLA, Cedars Sinai Medical Center). The study will be performed under the guidance of Principal Investigator Dr. Rona Shofty, DVM, PhD, DLAM.
The study as shown will demonstrate the ability of the Huygens™ Catheter to generate clinical data that is superior to the existing art, thereby demonstrating improved clinical indices in electrophysiological therapeutic procedures. Specifically, the study is designed to demonstrate the ability of the Huygens™ Catheter to perform detailed mapping and Dr. Eli Gang acquisition procedures in all four chambers of the test subject’s heart. Success in this study will constitute proof of design adequacy and equipment safety in reaching the efficacy and safety goals established in the study protocols.
A separate agreement is being finalized with Sandia National Laboratories to perform real-time simulation studies of the system under the guidance of Dr. Darren W. Branch, PhD., and is anticipated to proceed concurrently to demonstrate the embodiments that differentiate the Huygens™ Catheter from any other catheter in the marketplace today.
System Integration to be done at NKC Headquarters

NKC Validation Lab
In anticipation of the application approval, NKC expects to have the installation of its Huygen™ – Proteus™ Robotic Arm Surgical Platform completed at its lab facility in Los Angeles, California, by the end of October. Once completed, NKC will begin its own internal validation studies in preparation for the above trials.
The lab installation will allow the NKC engineering team the ability to test prototype designs, perform quality control checks, and establish operational and test protocols while gathering important analytical and comparative clinical data needed to further its regulatory approval strategy.
New NKC Patent Filing
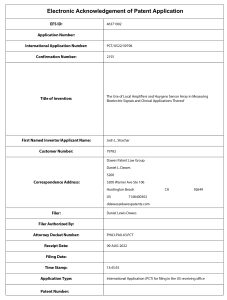
NKC Patent PCT/US2022/030399)
NKC has filed a new patent application developed by NKC’s CTO Josh Shachar. Titled, “The Use of Local Amplifiers and a Huygens Sensor Array in Measuring Bioelectric Signals and Clinical Applications Thereof” (PCT/US2022/030399), the patent relates to the field of electrophysiological mapping methods using the Huygens™ Catheter with its capabilities of co-measuring impedance and local native biometric signals, and employing such signals as well as its dynamics.
Clinically, this means the Huygens™ Catheter might solve one of the most fundamental
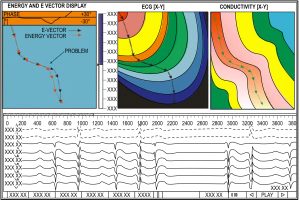
One of the advances enumerated in the patent, is the ability of the Huygens™ Catheter to accurately identify and measure phase singularities in order to provide a more accurate representation of the bioelectrical activity in the heart chamber.
problems in electrophysiology, and can then be used as a standard tool for care in treating pacing problems such as A-Fib and other disease models.
To date, NKC has filed eight patents, both domestically and internationally, describing its advanced catheter technology. This is in addition to the more than 30 patents and patent applications NKC has already filed in its IP strategy to both define and protect the company’s
growing advanced medical technology platforms.
General Integration of NKC Platform with Abbott/St. Jude EnSite NavX System
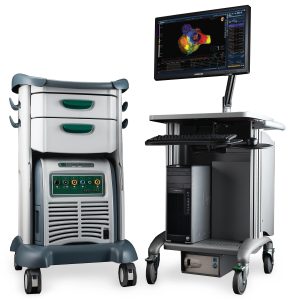
Abbott/St. Jude EnSite NavX EP Mapping System
NKC has entered into a collaboration with Abbott/St. Jude to integrate its Huygens™ – Proteus™ Surgical Platform with their industry-standard EnSite NavX EP mapping system, allowing data collected by the Huygens™ Catheter’s sensors to seamlessly integrate with the mapping system to create anatomical maps with dynamic range and resolution exceeding current standards; a parameter which will be qualified by NKC’s upcoming animal studies. The use of the EnSite NavX system with the Huygens™ – Proteus™ Surgical Platform will create faster high-density maps of any cardiac chamber while also providing the EP Surgeon information about the best diagnostic and ablation catheters to meet the needs of the patient.
From 9 Ton to 9Kg

CTO and Inventor Josh Shachar showing comparison of the 9kg Proteus™ Robotic Arm next to the 9 ton CGCI..
The Huygens™ – Proteus™ Surgical Platform represents two decades of work in advancing robotic catheter guidance from its original 9-ton flagship CGCI (Catheter Guidance Control & Imaging) Robotic System down to the 9kg device it is today.
CGCI was one of the first catheter guidance systems that used magnetic fields to navigate a specially designed catheter through a living heart chamber to provide interior tissue mapping for cardiac ablation procedures. The system utilized remote navigation using robotic control and artificial intelligence to maneuver the catheter tip exactly where the EP surgeon wanted. The system also integrated autonomous guidance for the ability to return a catheter automatically to a pre-mapped and targeted position.
The advances made with CGCI, and its early-development prototype the MOSFET Catheter counterpart have paved the way for the Huygens™ Catheter and the Proteus™ Robotic Arm reducing the technology that required a dedicated operating suite into a portable, plug and play system that can be deployed into any existing EP operating room while delivering detection sensitivity, mapping resolutions and AI enhanced guidance that can dramatically change the face of EP cardiac diagnostics and treatment.
Addition of Seasoned Regulatory Expertise to NKC Team
NKC has contracted two new team members to navigate the FDA regulatory pathway for product approval of the Huygens™ Catheter and the Proteus™ Robotic Arm technologies: Prof. Elaine Duncan (Paladin Medical Inc.) and Dr. Jaap Laufer MD (Emergo Group Inc.). Both of these highly qualified individuals bring to the table decades of experience in successfully guiding groundbreaking medical technologies to market.
Professor Duncan is the founder and president of Paladin Medical®, Inc. and is a certified regulatory affairs professional with a master’s degree in engineering. She holds an appointment as an Adjunct Professor of Biomedical Engineering in the F. Joseph Halcomb III, M.D. Department of Biomedical Engineering at the University of Kentucky and is a recognized leader in regulatory/clinical strategies for new medical technology development.
Dr. Laufer has over 30 years of regulatory and clinical experience specializing in implant, high-risk device and combination product submissions, FDA QSR compliance, and clinical study submissions and compliance. He has held corporate positions in Regulatory and Clinical Affairs for the Switzerland–based Lipomatrix, Pfizer Hospital Products Europe, and global giant Abbott Laboratories.
Together, along with Lead Regulatory Consultant, Susan Alpert MD, who has 30 years of experience and was the former Senior Vice President of Global Regulatory Affairs at Medtronic, and Quality Engineer Elissa Salceda, who has been hands-on in the development of the Huygens™ – Proteus™ Robot Arm Surgical Suite, NKC is on a solid path forward to achieving its first major commercialization milestone, FDA approval. This milestone will allow entry of the NKC technology into the U.S. marketplace. This will also provide benchmark clearance for the European CE Mark.

Prof. Elaine Duncan

Dr. Jaap Laufer MD

Dr. Susan Alpert

Elissa Salceda
Anacaz Group to Develop Digital Backend for NKC Clinical Data Management

NKC is in negotiations with Anacaz Networks Inc., to support the development of cloud-based operations and ensure security and safety of the preserved patient data acquired with the Huygens™ – Proteus™ Surgical Platform. This data will allow physicians to review the exact conditions and events recorded during a procedure including placement of ablative lesions, rotor locations, and the complete replay of actions taken by the surgeon. The establishment of this dedicated secure cloud platform will provide the ability for patient information and treatment to be accessible to the patient designated healthcare providers, as well as creating a growing database of information to be available for expanding the
knowledge base of cardio-disease treatment for EP physicians everywhere. Creating this secure central share-point is in alignment with NKC’s vision of helping to improve the EP art and to democratize medicine for everyone.

Rob abrams
About NKC
Neuro-Kinesis Corporation is a medical technology company focused on creating next-generation surgical tools incorporating advanced biosensor systems that can provide real-time biofeedback of a variety of process-critical data to a physician or surgeon where the monitoring of precise environmental status points can greatly enhance a patient’s procedural outcome.Interest in the NKC surgical tool technology has already been generated in several large medical device companies, and the company is on track to complete the continued prototyping and research studies required to bring their initial product candidates to pre-market commercialization.
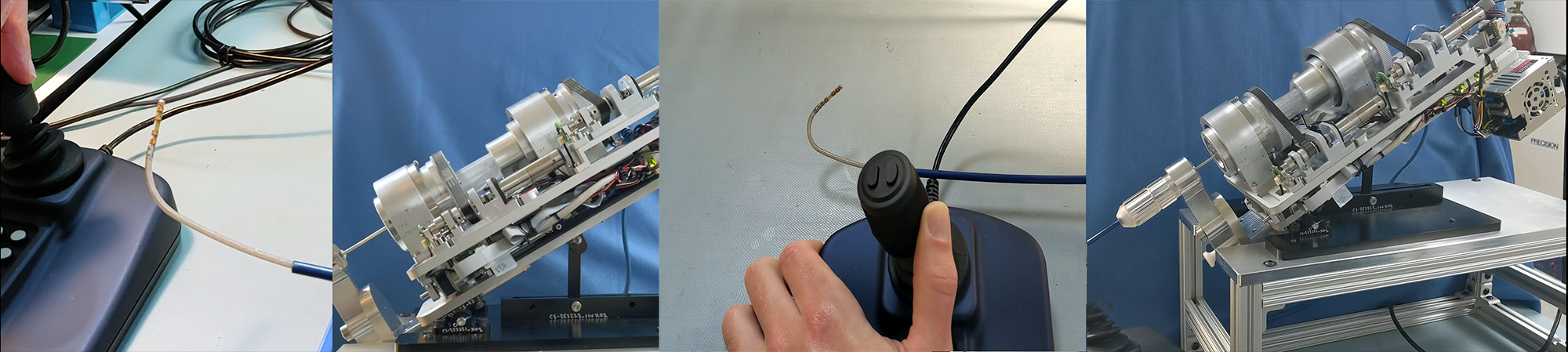
The images above show the Huygens™ – Proteus™ Robotic Arm Surgical Platform in development trials at NKC. From the left, the first two images show the Huygens™ Catheter being “steered” by the Proteus™ Robotic Arm. The Proteus™ is capable of moving the catheter in five degrees of freedom in any of the endocardial spaces. The third images shows a prototype joystick allowing effortless navigation control of the catheter by the EP surgeon. The final picture shows the Proteus™ Robotic Arm and part of the Proteus™ Catheter Handle being tested to confirm full rotational, transitional and deflection movement control. The above constitutes the full system.

To learn more about the PFDMOF3001
CLICK HERE

To learn more about Neuro-Kinesis Corp.
CLICK HERE
CONTACT
PFD Capital Partners
23161 Lake Center Dr. Ste. 100
Lake Forest, CA 92630
Email:
Office Phone:
(888) 475-4748
Fax Number:
(888) 475-4748


