PFDMOF3001 News
The following articles and press releases are to provide information on the current status of the PFDMOF3001 Opportunity Fund as well as the related technology companies in the portfolio.
If you have any questions regarding any information you find here, please contact us at: info@PFDCap.com

NKC Announces Upcoming Animal Study for Clinical Validation of its Huygens™ – Proteus™ Robotic Arm Surgical Platform
PFD shares this press release from Neuro-Kinesis Corporation, one of the medical technology comanies in its PFDMOF3001 Opportunity Fund portfolio which discusses the NKC’s announcement of two upcoming studies to be performed at the Technion Institute in Israel and at Sandia National Laboratories. Each of these studies are being done to validate the technology behind its Huygens™ Catheter platform.

World Health Organization Says “We’re in a Wound Pandemic”
PFD share information which relates to its partnership with CassdermaRx, one of the portfolio companies in the PFDMOF3001 Opportunity fund. The article published in the C-Street Advisors regards a recent study by the World Health Organization and the National Institutes of Health examining the rising concern over recalcitrant wound growth statistics.

Introducing Dr. Daniel Rastein, Cassderma RX and Castle IRB
In this release, PFD shares an update on Dr. Daniel Rastein, MD, MPH who is the Founder & CEO of Cassderma RX, one of the portfolio companies in the PFDMOF3001 Opportunity Fund. Dr . Rastein has been retained by the PFD team to coordinate the Institutional Review Board Sponsored by Advarra for the PFD/iOrthopedics INC new orthopedic Resilient Arthroplasty Device (RAD) referred to as the iKnee,
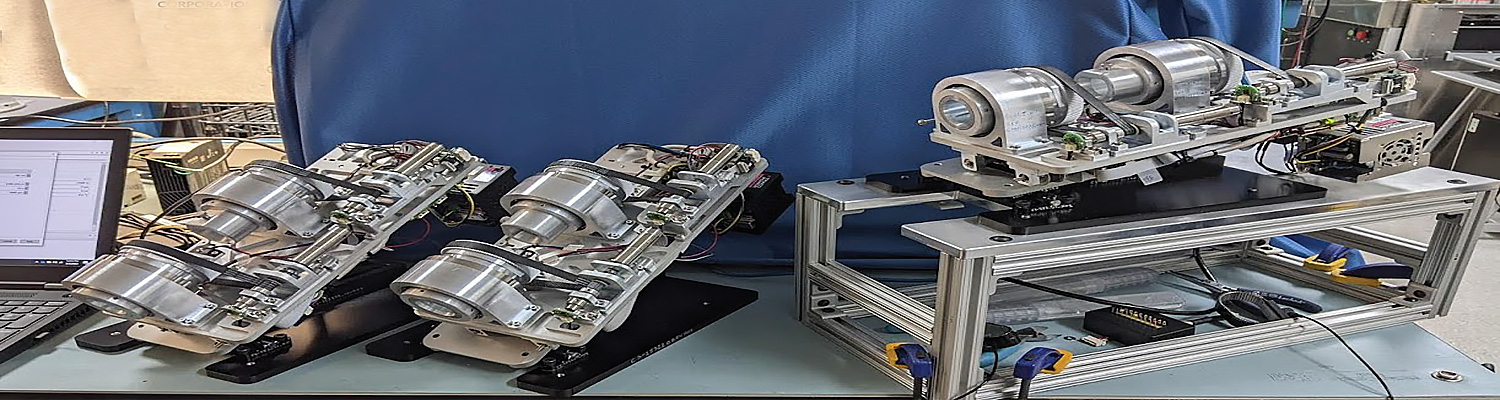
Phase One Development of Huygens™ Smart Catheter & Proteus™ Robotic Platform Completed
PFD is pleased to share this press release from Neuro-KInesis, Corp. (NKC), one of the medical technology companies in the PFDMOF3001 Opportunity Fund. In the release NKC announced they have completed the Phase One development for their new Huygens™ Smart Catheter and Proteus™ Robotic Arm platform as well as five other strategic goals towards its efforts to bring its patented EP Catheter Mapping and Guidance Technology to the Electrophysiology marketplace.

Managing Medical Receivables In Healthcare
In this article PFD shares important information on what Medical Receivables (MR) are and why careful management of these assets is the lifeblood of any medical practice. PFD Capital Partners, LLC not only is in the business of helping healthcare providers in removing difficult to collect MR Personal Injury claims from the ledgers, but we also work to help medical professionals in understanding suggested best practice ideas for streamlining the PR process.

The Need For Greater Transparancy In Medical Receivable Funding
In this articles, PFD shares some important information on how the rise in medical receivables financing has created many bad actors in the space and why the importance of making sure the company you decide to do business with practices the highest ethical standards and offers the greatest transparency on how it does business.

Creating A Method For Assessing Recovery From Orthopedic Knee Treatment
PFD share a recent study by researchers from the University of Seville and the University of Cadiz in Spain indicates that there is currently no standardized methodology for qualitative and quantitative for assessing the recovery of patients who have undergone orthopedic knee treatment. This relates to the work being done by PFD/iOi in creating its Resilient Arthroplasty Device known as the iKnee.
