Introducing Dr. Daniel Rastein, Cassderma RX and Castle IRB
PFD MANAGEMENT
OPPORTUNITY FUND 3001, LLC
23161 Lake Center Drive, Suite 100
Lake Forest, CA 92630
Telephone (661) 665-6074, Toll free (888) 475-4748
FOR IMMEDIATE RELEASE
DATELINE: March 29, 2022 Lake Forest California
This Release relates to Dr . Daniel N. Rastien, PFD FDA IRB Program Coordinator. In addition to being retained by the PFD team to coordinate the Institutional Review Board Sponsored by Advarra for the PFD/iOrthopedics INC new orthopedic Resilient Arthroplasty Device (RAD) referred to as the iKnee, Dr. Rastein, MD, MPH is also the Founder & CEO of Cassderma RX, one of only four portfolio projects selected as meeting the exacting standards of PFD Capital Partners for the PFD Management Opportunity Fund 3001, LLC.
ABOUT CASSDERMA RX
Cassderma RX is the developer of an innovative pharmaceutical-grade topical gel that supports the rapid regeneration of tissue for atrophic recalcitrant wounds. The gel is based on a patented formulation that uses a serum-free, nutrient-rich medium supplemented with a non-steroidal anabolic hormone to stimulate and support the growth of connective tissue originating from a wound bed and its peripheral walls.
Founded in 2020 to begin the process of bringing to market an innovative discovery in a curative wound healing topical product, Cassderma RX is a direct result of the decade-long work of Dr. Daniel Rastein.
As an educator and noted industry leader in healthcare and biomedical research, Dr. Rastein has been involved in designing the regulatory standards of numerous drugs and biomedical devices for cutting-edge manufacturing, research, medical, aerospace, and agriculture companies.
In 2010, Dr. Rastein became aware of an abandoned patent for a unique wound-care formulary. Because of his background in bioresearch, Dr. Rastein immediately saw how important the product that was described would be in presenting a game-changing shift for the treatment of chronic wound-care patients. During the next ten years, Dr. Rastein worked with a key group of formulary researchers, patent lawyers, and pharmaceutical marketing experts to take the concepts from the abandoned patent in order to secure the rights and revive the patent in order to bring this work from vision to reality.
Because of the dedication of Dr. Rastein and his team, a new patent was able to be granted providing four unique allowances, two for formulary and two for application methods, which cleared the road to begin the next steps for regulatory approval.
The result of this eff ort is the Cassderma RX Wound Healing Hydrogel which is a first-of-its-kind topical agent that delivers vital nutrients directly to a wound site. It also provides a naturally occurring hormone that stimulates the growth of healthy cells at the wound site to promote the regeneration of connective tissues vital to the restoration of new skin and deep tissue to heal a variety of wound types including those from pressure wounds, diabetic ulcers, as well as burns and surgical wounds, which are some of the most di cult to cure.
Cassderma RX is now focused on moving the invention through the necessary Clinical Trials to gain an FDA Investigational New Drug Authorization that would allow it to begin human clinical trials.
About Castle IRB (the sponsor)
Castle IRB is a premier institutional review board o offering IRB review services in many areas of research with a specialization in genes, cell therapy, and rare disease space.
What is an IRB?
Institutional Review Boards, or IRBs, review research studies to ensure that they comply with applicable regulations, meet commonly accepted ethical standards, follow institutional policies, and adequately protect research participants.
What is its purpose?
Under FDA regulations, an IRB is an appropriately constituted group that has been formally designated to review and monitor biomedical research involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, require modifications (to secure approval), or disapprove research.
CASTLE IRB NEWSLETTER
VOLUME 2

MELISSA FINK
Director of IRB Operations
MESSAGE FROM THE DIRECTOR OF IRB OPERATIONS
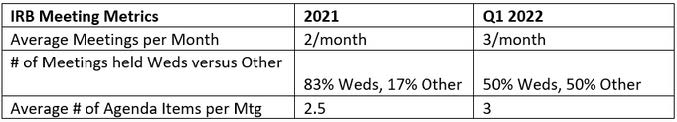
Greetings Castle IRB Members! It is hard to believe that we are nearly a quarter into 2022 already. This issue, I wanted to share some metrics on full board operations, and to give you a glimpse into the goals we’ve set for full board review operations this year.
As you can see by the IRB Meeting Metrics table below, volume is increasing, and to meet turnaround time (TAT) commitments to our clients, half of the meetings convened in Quarter 1 of 2022 were moved to a nonstanding day. Given this, Castle will be starting off Q2 with adding a second standing meeting a week. Thank you all for sharing your availability for the selected time: Fridays, 10am-12pm Central. Those who indicated availability, please begin holding that time on your calendar beginning in April; as usual, invitations to attend any given meeting will come a week in advance.
Due to the increase in volume, we’ll also be looking at adding Vice Chair roles to the Castle IRB committee to help chair meetings. We’ll also be introducing additional tools and training targeting increased efficiency in how we operate and consistency in our reviews. Hopefully all changes that make our roles clearer as we each have a unique set of expertise to bring to reviews (more from our Chair Stephanie on that, below).
The first efficiency initiative we are taking up is related to IRB Meeting Minutes, and with this we need your help! Castle IRB is aiming to shorten the timeline from IRB Meeting to finalized minutes to 3 weeks. To accomplish this, we must shorten the timeframe for IRB Member review of draft minutes to 3 business days and request signatures on the final draft within 2 business days. Thank you all for your help with this goal!
We are experiencing 100% client satisfaction right now, and our network is growing. We appreciate your flexibility and help in refining and improving our full board operations as we grow!

Dr. Stephanie Solomon-Cargill
IRB Chair
MESSAGE FROM THE IRB CHAIR
In this letter, I hope to take a moment to discuss the expectations and roles of the different board members in their reviews. Hopefully this will illuminate ways you can approach your reviews to tailor them to your unique backgrounds and perspectives to enhance your contributions to the review process.
Federal regulations dictate that IRBs must be composed of no less than five members with varying backgrounds and expertise in order to provide an adequate review of the research and to promote respect for its advice, counsel and determinations. More specifically, they dictate that members of IRBs:
- come from more than one profession
- have at least one member whose primary concerns are inscientific areas
- have at least one member whose primaryconcerns are in non-scientific areas
- have at least one member who is not affiliated with theinstitution (in our case, Castle, Sabai Global, or any of itssubsidiaries).
The spirit behind these requirements is the recognition that in order to adequately evaluate the ethics of human subject research, IRBs require numerous types of expertise and perspectives to adequately assess whether the criteria for approval has been met for each study.
Here at Castle IRB, we apply these regulations and incorporate more members trhan required to specifically address the types of research we encounter. Sometimes board members can satisfy more than one of these roles,and as such should look at the research from multiple perspectives.
Reviewers designated as scientists/medical experts use their expertise and ability to look at the the study to make sure that it is designed in such a way that it will answer the question it is asking, that the risks are minimized, and that the consent documents accurately convey what is happening in the study. This includes making sure that the significant and common risks of the study are included in the consent.
Reviewers who represent the perspective of research participants use their expertise about the experience of those being recruited into studies. They are expected to look at the study to make sure that the burdens to participants are recognized and minimized, that the consent conveys information clearly that participants would find important and relevant to the decision to participate, and to continue participating. Finally, that the board can see more broadly how the study will be received by participants in their particular contexts.
Reviewers who represent non-scientists look primarily at the consent and other participant-facing materials to ensure that the content, language and formatting used is likely to be understandable to people without a scientific background. Specifically of concern are medical terminology that is without lay language explanations, explanations of concern to participants that may be absent or unclear (ie.insurance coverage), and terminology that may be perceived as offensive or insulting from a lay person’sperspective.
Reviewers designated as un-affiliated with Castle, Sabai Global, or any of its subsidiaries review the study with the perspective of someone who doesn’t have a primary concern for Castle IRB as a business, or those who submit to us as clients. This is intended to serve as a check so that the focus on serving our clients cannot distract us from our priority to respect and protect research participants in the studies we review.
Reviewers who are designated as a Gene Therapy Scientist ensure that we have the perspective and knowledge of biosafety review and the complex science involved when we review gene therapy research.
Reviewers who are designated as Regulations experts, usually one of the staff, ensure that we are looking at the studies with an eye to any regulatory or legal requirements that must be satisfied in them.
Finally, many of you have perspectives in addition to these designated ones that provide you with insight and expertise in areas that are vital to the review process. This could come from your career backgrounds, your backgrounds as parents or family members, your backgrounds with certain communities, populations, or other areas. All of these perspectives are what make working together on an IRB such a fascinating and productive process, and ultimately serves the participants who are recruited for our studies with the best ethical review.
Thank you all for your service and your perspectives!
CURRENT NEWS and UPDATES
In the News:
- Last month the U.S. Patent and Trademark Office ruled on a patent dispute about who shouldown the patent for first developing CRISPR-Cas9 gene editing technology.
- FDA approved Carvykti as a new CAR T-cell treatment for patients with elapsed/refractorymultiple myeloma who have previously tried at least four therapies. The therapy, developed byJanssen and Legend Biotech, is the third approved CAR T that targets B-cell maturationantigens (BCMA) and is the sixth genetically-modified cell therapy to win approval in the U.S.
- FDA Final Guidance MAR 2022: Expansion Cohorts: Use in First-In-Human Clinical Trials toExpedite Development of Oncology Drugs and Biologics Guidance for Industry
- FDA draft guidance open for public comment Mar 2022: Considerations for the Developmentof Chimeric Antigen Receptor (CAR) T Cell Products and Human Gene Therapy ProductsIncorporating Human Genome Editing.
WEBINARS and EVENTS
WEBINAR:
 NYU Langone Health Working Group on Pediatric Gene Therapyand Medical Ethics (PGTME) 2nd annual lunchtime lectureseries, Critical Discussions: Multistakeholder Perspectives on the Ethics of Pediatric Gene Therapy Research
NYU Langone Health Working Group on Pediatric Gene Therapyand Medical Ethics (PGTME) 2nd annual lunchtime lectureseries, Critical Discussions: Multistakeholder Perspectives on the Ethics of Pediatric Gene Therapy Research
Topics include:
- Pediatric Gene Therapy Research in the Context of UncertainRisk-Benefit
Trust & Transparency for Trial Participants and Families - The Many Facets of Hope
- The Lived Experience: Social, Emotional, and Practical Aspects
- Accountability and Collaboration with Patient Communities
 Upcoming Free Event: Intro to Viral Vectors:
Upcoming Free Event: Intro to Viral Vectors:
Thursday, Mar 31 12-1 CT
During this free virtual event, experts in thefield will dive further into viral vectors, a common deliveryapproach used in gene therapy. Learn more click here.
Past Webinars of Interest:
Gene Therapy 101 webinar
ASGCT’s Q4 2021 Gene, Cell, and RNA Therapy LandscapeQuarterly Data Report
MEMBERS CORNER
 Spotlight: Daniel Rastein
Spotlight: Daniel Rastein
How did you start your career in clinical research?
My career in clinical research actually started in 2005 with pre-clinical research when I was assigned to make the research department at Cedars-Sinai Medical Center, GLP (Good Laboratory Practice) complient. I was familiar with GCP (Good Clinical Practice) from the human side, but I had no idea what GLP was, especially when I showed up for work in the vivarium! As you can imagine, the sights, sounds and smells were very different than the main medical center. Although shocked at first, being an animal lover, I quickly fell in love with the extreme caring nature of the dedicated staff and all of the rules and regulations to protect and reduce harm to the most vulnerable of our society. I used this strong foundation every day in ethical discussion in clinical research to this day. I also still give lectures on GLP to the research community.
 If you could trade places with anyone for a day, who would it be and why?
If you could trade places with anyone for a day, who would it be and why?
If I could go back in time, instead of trading places, I would love to shadow, assist and learn from Mother Teresa. She would seek out and approach the poorest and sickest people living in the slums and say “I love you and want to take care of you.” This unconditional love and service towards mankind is definitely something that I aspire to.
What’s a fun fact about you that not many people know?
I am an active participant in the James Beard Foundation. The foundation is a nonprofit organization whose mission is to celebrate, support, and elevate the people behind America’s food culture and champion a standard of good food that is anchored in talent, equity, and sustainability. One of the programs that I am most excited about is called “Good Food For Good.” That offers educational scholarships in the culinary field as well as educational programs that teach food sustainability as well as providing support to struggling restaurants and their workers through these very challenging times.
What is the weirdest food you have eaten (or a favorite food)?
Being a foodie, I am always adding new spices to my collection as well as exploring different cultures. I remember being invited to try something different by some Korean friends and neighbors. Of course I rushed over to their house all excited to try whatever it was. Once seated, they placed in front of me a giant jar filled to the rim with what looked like soy sauce. When it was opened, the contents had a very unique smell, which my hosts identified as Gejang. I was a bit confused until they reached inside and pulled out a big raw whole crab.They thought that I would be deterred, but I dove right in. It was tasty once you get over the texture of the raw crab meat, which was a little gelatinous and slimy. They were surprised as evidently, according to them, it was an “advanced” food that most non-Koreans did not enjoy.

To learn more about the PFDMOF3001 Opportunity Fund
CLICK HERE