PFDMOF3001 News
The following articles and press releases are to provide information on the current status of the PFDMOF3001 Opportunity Fund as well as the related technology companies in the portfolio.
If you have any questions regarding any information you find here, please contact us at: info@PFDCap.com

PFD Management Shares Update PFD/iOI’s FIH Implant
PFD/iOI announces it has cleared the final hurdles in order to move ahead with its First-In-Human implant of its RAD/iKnee technology.

PFD Management Shares Q123 Clinical Progress and Update On CassdermaRx
The FDA and HHS release report discussing the need for better care protocols for patients suffering from chronic wound issues. This support PFD’s efforts with CassdermaRx, one of the medical technology companies in the PFDMOF3001 Opportunity Fund portfolio.

PFD iOI Announces Update On The iKnee Comfort Study And The Path Forward
PFD/iOrthopedics, Inc. (PFD/iOI) has announced further details on its upcoming iKnee Comfort Study.

PFD iOI Announces Updates On The RAD/iKnee Lab Installation
PFD/iOI has released an update on the installation of its ARBURG Freeformer 300-3X 3D printer into the PFD/iOI RAD iKnee Lab at its Los Angeles facility.

PFD iOI Announces Acquisition of Arburg Freeformer 300-3X For Production Of iKnee
PFD/iOI has announced the purchase of the Arburg Freeformer 300-3X 3D printer to start prototype development and manufacture of its iKnee technology. The printer is to be installed at PFD/iOI’s new clean room lab at its Los Angeles facility.

PFD Management Shares End of Year HHS Public Access Author Manuscript Recognition from CassdermaRx
The FDA and HHS release report discussing the need for better care protocols for patients suffering from chronic wound issues. This support PFD’s efforts with CassdermaRx, one of the medical technology companies in the PFDMOF3001 Opportunity Fund portfolio.
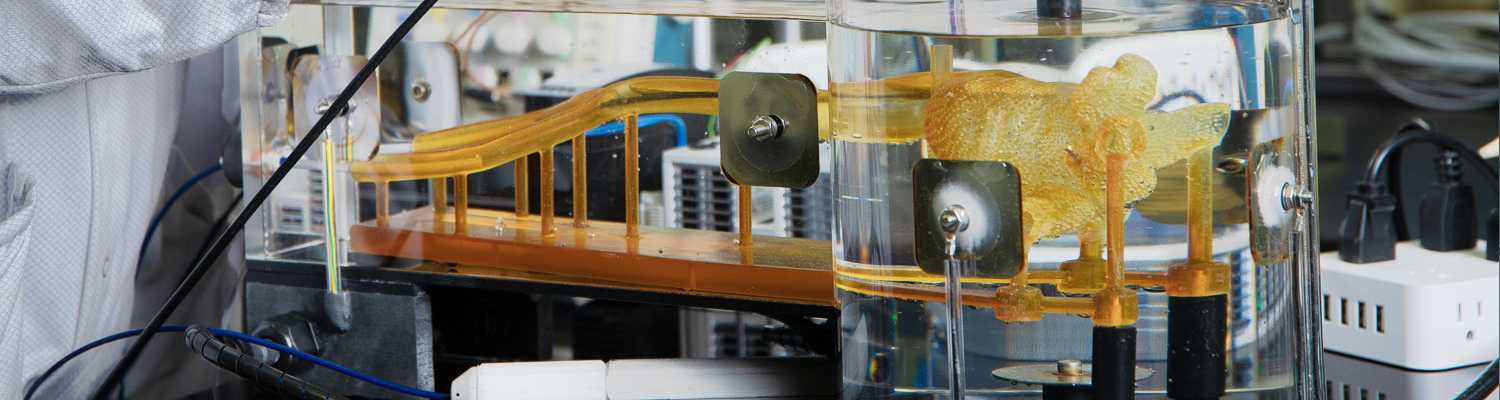
PFD Management Shares NKC End-Of-Year Development Report
PFD is proud to share this end-of-year update on Neuro-Kinesis Corporation which is one of the featured medical technology companies in the PFDMOF3001 Opportunity Fund portfolio.
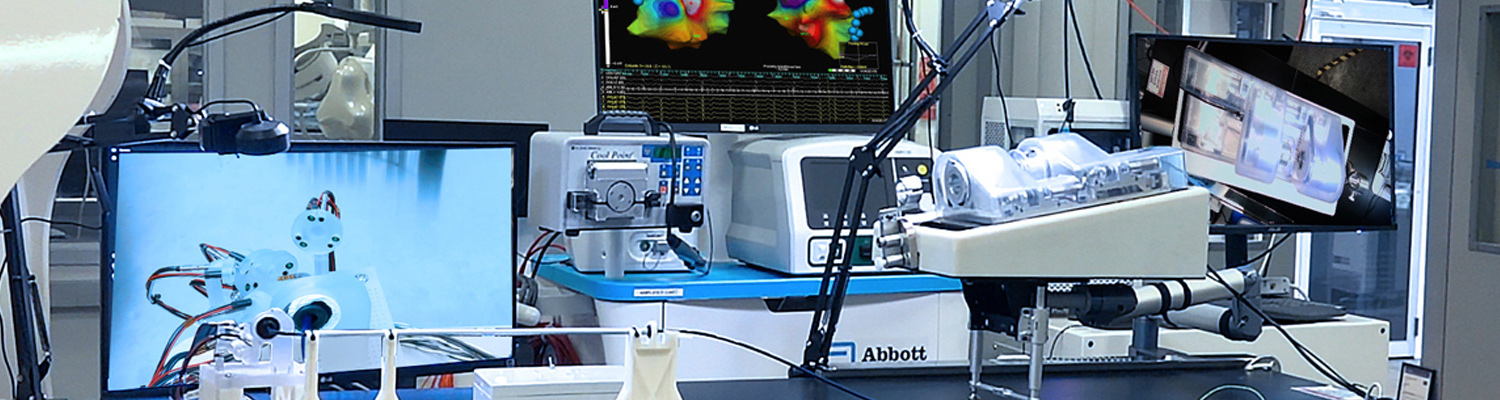
PFD Management Opportunity Fund 3001 Press Release November 15, 2022
PFD is proud to share Neuro-Kinesis Corporation’s fourth quarter 2023 update on it Huygens™ Catheter and Proteus™ Robotic Arm Technologies. this end-of-year update on Neuro-Kinesis Corporation is one of the featured medical technology companies in the PFDMOF3001 Opportunity Fund portfolio.

PFD/iOI Release Update On Their Resilient Arthroplasty Device (RAD) Technology
PFD is pleased to share this updates on its PFD/iOi venture. This includes information of the latest design enhancements by our engineering team for the Resilient Arthroplasty Device – iKnee. as well as information the finalized prototype design, and the initial Proof of Concept with First In Human implant and the launch of the iKnee Comfort Study.

NKC Release Third Quarter Development Report on the Huygens – Proteus™ Robotic Arm Surgical Platform
Neuro-Kinesis Corporation has released its Third-Quarter Development Report on its Huygens™ Catheter and Proteus™ Robotic Arm Technologies. which is one of the featured medical technology companies in the PFDMOF3001 Opportunity Fund portfolio. The report includes information on the company’s application for Institutional Animal Care and Use Committee Approval (IACUC) for its upcoming animal studies as well as its agreement with Abbott/St. Jude for use of its EnSite NavX System in the NKC Operating Suite.
