
about
Neuro-Kinesis is an advanced medical technology company focused on creating next-generation surgical tools that incorporate innovative biosensor technologies with Artificial Intelligence that dramatically advance a physician’s ability to provide enhanced patient outcomes.
about
Neuro-Kinesis is an advanced medical technology company focused on creating next-generation surgical tools that incorporate innovative biosensor technologies with Artificial Intelligence that dramatically advance a physician’s ability to provide enhanced patient outcomes.
background
NKC was founded in 2016 to capitalize on the 15-year work of the engineering team in developing smart medical devices that would integrate their proprietary biosensor technology for targeted real-time biometric feedback with advanced AI analysis and wireless cloud connectivity into high-need surgical tools that could fundamentally change the ability of a physician to treat specific disease models. Before the formation of NKC, the team had achieved tremendous success with their revolutionary robotic-assisted catheter guidance system (CGCI) and with the development of the first portable pathogen detection system that brought scientifically relevant detection results in minutes rather than days or weeks and did so at a fraction of the cost of normal detection laboratories. This latter technology was recently acquired by Autonomous Medical Devices (AMD) which is owned by Oracle founder, Larry Ellison.
vision
NKC’s vision was created and continues to be spearheaded by inventor, scientist, and entrepreneur Josh Shachar. Josh’s work in guidance and navigation systems for the Department of Defense forms one of the bedrock keys for global defense as componentry developed by his aerospace companies exists in virtually all free world missile programs operating today. For the past 20 years, Josh has turned his focus to defining and solving critical engineering and physics problems related to advanced medical devices for disease mitigation. He currently holds more than 100 patents related to his pioneering work in robotic guidance, smart drug delivery, pathogenic biomarker detection, and SMART medical tool development. His vision continues to break new ground and advance medical discovery.
innovation
NKC is the developer of several innovative medical device technologies that are each designed to lessen the limitations that are imposed by outdated instrumentation and methodologies faced by physicians and healthcare providers.
CGCI
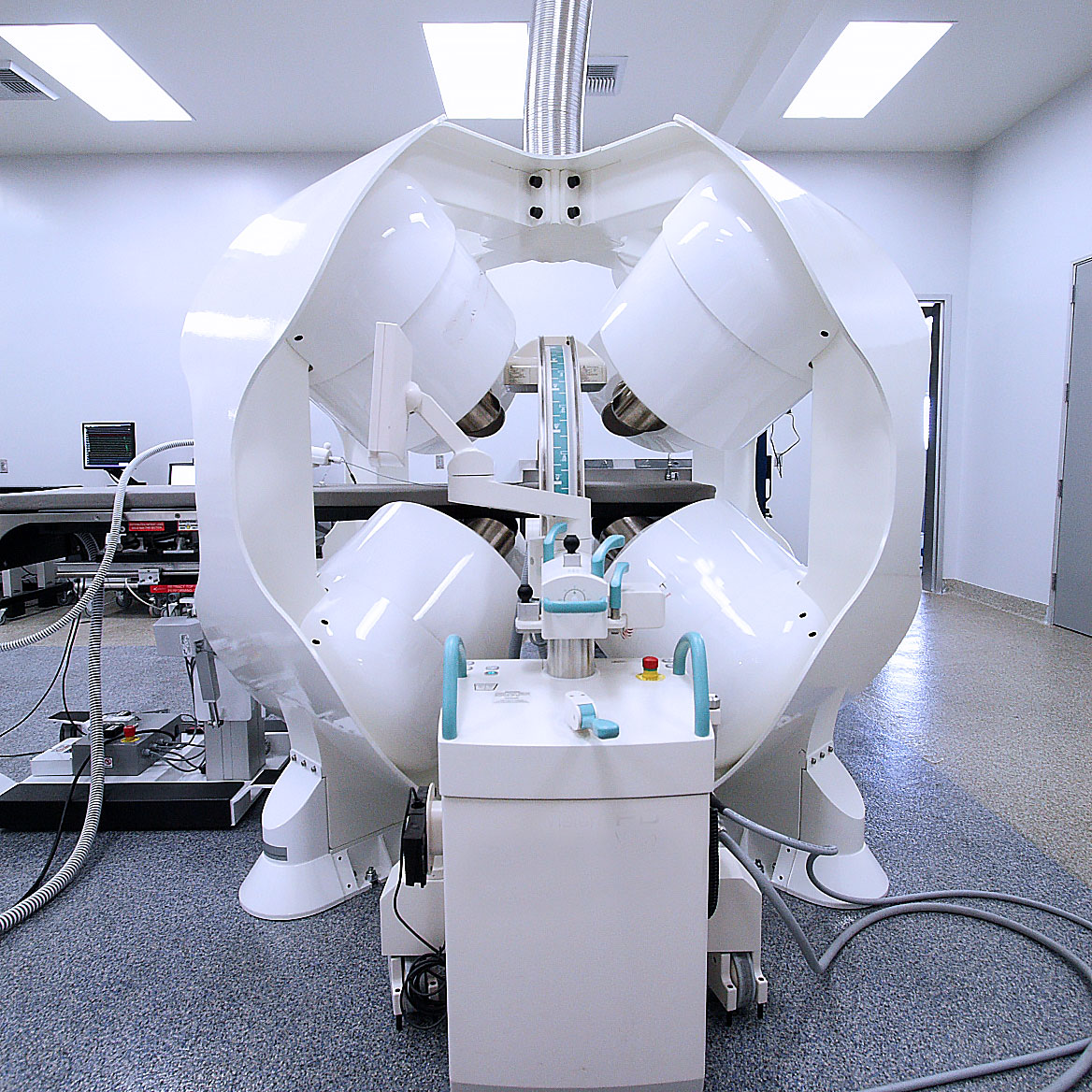
The Catheter Guidance Control and Imaging (CGCI) system was developed by Josh’s engineering team in 2008. The system was created to provide a way to navigate a catheter inside a human body using magnetic force and robotic guidance systems. In addition, the CGCI was able to provide a 3D mapping of the interior of the beating heart to provide the surgeon the ability to mark and define their ablation points. Since the system was robotically controlled, once positions were targeted, the CGCI could return a catheter to any position automatically at the push of a button.
OVERVIEW
CGCI (Catheter Guidance Control and Imaging System) was created to solve two of the main issues associated with catheter navigation and placement in the field of Electrophysiology. The first is the variability and difficulty of placing and moving a catheter accurately within a beating human heart for the treatment of conditions such as aFib where such movement is entirely dependent upon the dexterity of the physician pushing and pulling on a three-meter-long catheter from outside the patient’s body. The second issue is the ability of a catheter to accurately map and visualize the tissues of the heart to correctly identify where ablation points need to be executed.
CGCI uses eight powerful magnetic lobes that can guide a specially designed catheter tip in any X, Y, and Z orientation inside the human body, and specifically within the heart. By varying the magnetic field of each lobe, exact movement and placement can be made. The operating surgeon uses a Haptic controller to send catheter control motions which the system’s robotic control immediately translates to the magnetic lobes.
A special catheter tip provides the surgeon a high-resolution 3D map of the interior of the heart whereby they can identify the electro-potential of the tissue to ascertain where ablation points need to be executed.
Once the points have been mapped, the surgeon can remove the catheter and replace the tip with an ablation catheter and then reposition the catheter back into the heart chamber. At this point, the surgeon can then initiate the pre-programmed ablation routine and the CGCI’s robotic system automatically performs the required procedure.
CERTIFICATIONS
The CGCI platform has received many certifications of its technology validating its capabilities and safety.
 |
The UL mark certifies that CGCI has been thoroughly tested for scientific safety, quality, and security standards by the US Government. |
 |
IEC 60601-1 certifies the basic safety and essential performance requirements of a medical device meets required single electrical, mechanical, and functional requirements and does not pose an unacceptable risk to patients and/or operators. |
 |
IECEE CB is an international scheme for mutual acceptance of test reports and certificates covering the safety of electrical and electronic equipment, devices, and components. |
 |
The CE mark certifies that a product sold in the European Economic Area has been assessed to meet high safety, health, and environmental protection requirements and can be sold and operated in those areas. |
 |
The KFDA Certification recognizes that the CGCI platform has been tested and reviewed by South Korea’s Ministry of Food and Drug Safety and is safe to be installed and used in Korea. |
INSTALLATIONS
CGCI has been installed in five separate locations around the globe including:
- Magnetecs Headquarters – Los Angeles, CA USA
- Cedars-Sinai Medical Center – Los Angeles, CA USA
- La Paz University Hospital – Madrid, Spain
- Na Homolce Hospital – Prague, Czech Republic
- Yonsei Severance Hospital – Seoul, South Korea
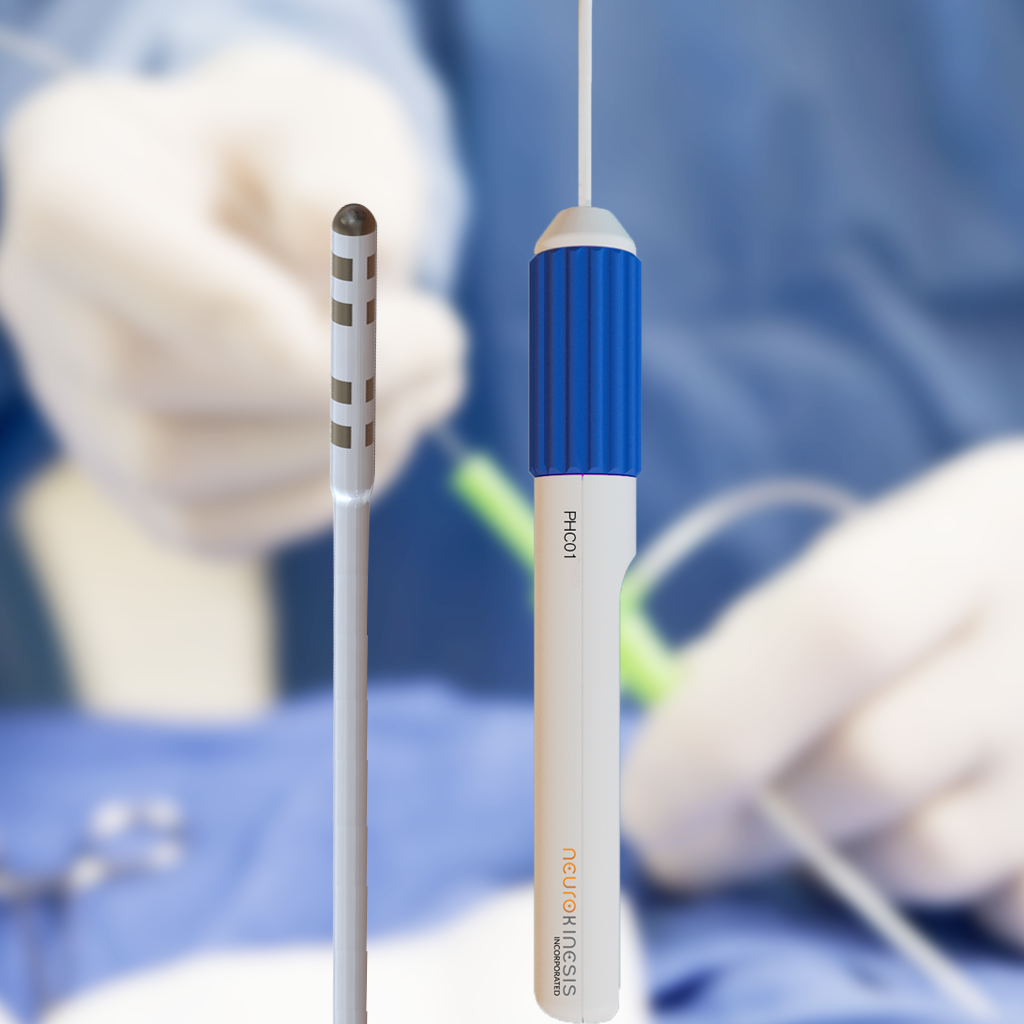
HUYGENS™ CATHETER
During the development of CGCI, the NKC Engineering Team discovered that the available technology for cardiac tissue mapping was severely lacking in many areas. The two primary challenges were the ability for a catheter to be able to “see” relevant information from what was just environmental noise. The second was the ability to detect the bioelectric information at a far higher resolution to allow for much more granular discernment of the health of the cardiac tissue. The Huygens™ Cartheter represents almost 10 years of research and development in creating a next-gen mapping catheter that takes EP heart mapping to a whole new level.
OVERVIEW
Accurate cardiac mapping is essential in the field of Electrophysiology to provide a surgeon the ability to identify the exact electrical potential, or ability to conduct electrical signals, that specific areas of the heart have so that corrective actions, such as ablation surgery, can be performed to correct heart pumping issues such as AFib or other arrhythmia-based diseases.
Mapping catheters work by reading the electrical conductivity of a point of tissue it comes in contact with. The ability to accurately determine the tissue’s bioconductivity is based on the ability of the catheter electrode to distinguish the tissue’s electrical signal from the wall of noise that surrounds it. In the chamber of a heart, inside a modern operating room, small bioelectric signals can be completely washed out by environmental noise so at best, the surgeon is only able to pick out the strong electrical signals, and not the smaller ones. Ones that would better inform the diagnostic procedure.
The Huygens™ Catheter addresses and solves this problem by moving all the signal gathering and analog interpretation of heart mapping, from the distal end of the catheter, which is generally inside a machine more than 6-10 feet away from the catheter tip, to an embedded architecture that resides on the catheter tip electrode itself.
Through proprietary miniaturization and algorithmic processes, NKC has been able to create a catheter electrode that can gather relevant electrode biopotential readings at a level 200 times smaller than current technologies, and then correlate and process that analog data and convert it immediately to digital data that is incorruptible, which is then sent back to the surgeons monitoring station.
This breakthrough now provides the EP physician with a level of resolution and 3D heart mapping that is completely unprecedented.
FEATURES
-
- The Huygens™ SMART Catheter can be fitted to almost any standard catheter handle.
- The catheter uses nine specially designed mapping electrodes in an array at the catheter tip.
- The catheter is able to accurately measure signals less than 10μV
- Signal-to-noise ratio filtering is greatly improved through a series of proprietary filters and pre-amps.
- All processing componentry is built onto a flexible micro-ship board inside the catheter tip.
- 12bit analog-to-digital conversion provides greater resolution than current standards provide.
- Once in a digital format, this incorruptible signal is then transmitted via fiber-optic cable to the mapping station.
- Latency from signal measurement to signal reading display is almost zero, providing real-time fast mapping of the heart to be performed.
- When combined with the Proteus™ Robotic Arm and the Proteus™ Handle, the Huygens™ SMART Catheter’s movement and control can be completely automated.
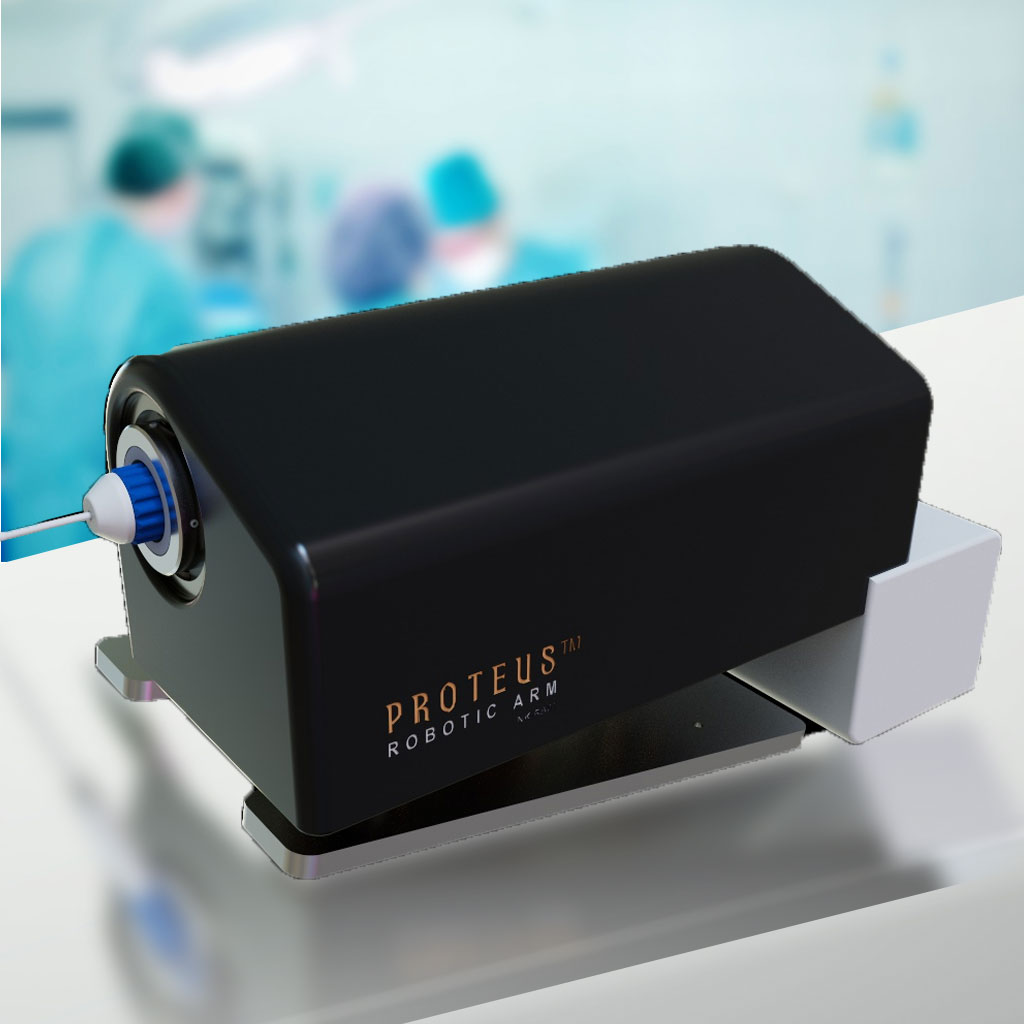
PROTEUS™ ROBOTIC ARM
The Proteus™ Robotic Arm takes all the engineering breakthroughs of the CGCI system and delivers robotic-assisted catheter guidance into a device that is no bigger than a shoebox. When combined with the Huygens™ Catheter, the Proteus™ Robotic Arm gives an EP surgeon a whole new level of control in performing heart mapping and ablation procedures. Because of its built-in robotic control and AI, the Proteus™ is able to provide touch-of-button return to target, and execution of ablation protocols.
OVERVIEW
The Proteus™ Robotic Arm provides the EP Surgeon with a portable benchtop ability to bring robotic-assisted guidance to catheter navigation inside the dynamic environment of the human body. Coupled with NKC’s Huygens™ Catheter, and the NKC Control Suite, the Proteus™ allows a surgeon to not only robotically move a catheter in any axis of orientation with a finer degree of control than can be done manually, but also the ability to map and recall those movements at the push of a button. This allows an EP Surgeon to be able to instantly return to a marked position as well as the ability to execute a pre-programmed point-to-point navigation path.
FEATURES
-
- The Proteus™ Robotic Arm brings the control and guidance capabilities created with the almost nine-ton, $2m CGCI system into a portable device the size of a shoebox and with a cost less than $150K.
- The Proteus™ Robotic Arm provides complete control of a catheter up to ± 2mm degree of accuracy in any X, Y, or Z axis of orientation as well as control of 360° deflection of the catheter tip.
- The Proteus™ Robotic Arm can control any standard catheter handle, but when coupled with the Huygens™ Catheter, the complete robotic navigation and mapping potential of the system is unleashed.
- Control of the Proteus™ Robotic Arm and the Huygens™ Catheter is managed by the NKC Guidance System that works in conjunction with the NKC Mapping Station which can be located immediately next to the operating table.
- An advanced Haptic Control device, which permits full axis directional movement, as well as force-feedback input of the tissue the surgeon is in contact with.
- The Proteus™ Robotic Arm can also be controlled automatically in response to the initiation of a course path that the surgeon has programmed into the system once the mapping and diagnostic procedures have been completed.
NKC and PFD
PFD Management has identified Neuro-Kinesis Corporation as one of the five Medical Technology companies to include in the PFDMOF3001 Opportunity Fund, LLC. PFD Management believes that Neuro-Kinesis Corp. with its Proteus™ Robotic Arm and Huygens™ Catheter; is on track to meet a significant commercialization event within the fund’s time frame. To learn more about the specifics of this component of the fund, register for access at our Membership Portal.




