PFD/iOI Release Update On Their Resilient Arthroplasty Device (RAD) Technology
PFD MANAGEMENT
OPPORTUNITY FUND 3001, LLC
23161 Lake Center Drive, Suite 100
Lake Forest, CA 92630
Telephone (661) 665-6074, Toll free (888) 475-4748
FOR IMMEDIATE RELEASE
DATELINE: September 20, 2022 Lake Forest California
PFD/ iOrthopedics Inc. (PFD/iOI) released new information regarding developments for its Resilient Arthroplasty Device (RAD), and its first product candidate the iKnee. In the report, PFD/iOI covers some of the latest milestones met in the prototype development of the iKnee as it approaches its first -in-human-clinical trial.
PFD/iOI is one of the medical technologies targeted by PFD Capital Partners, INC for its PFDMOF3001 Opportunity Fund, LLC (PFDMOF3001).
PFD Capital Partners, LLC Director and Fund Manager for the PFD Management Opportunity Fund 3001, LLC, Robert Pryke announced today additional points of interest to its portfolio project PFD/iOrthopedics, Inc.
PFD/iOI Release of Development Update on the Resilient
Arthroplasty Device (RAD) – iKnee
Lake Forest, CA: PFD Management/iOrthopedics Inc. (PFD/iOI) has updated its development status on the Resilient Arthroplasty Device – iKnee. The update includes the latest design enhancements by our engineering team at EnginuityWorks. The finalized prototype design, currently being reviewed by Dr. Thomas Grotz and Dr. Christopher Woodson, will be sent off to ArBurg for 3D Print and initial Proof of Concept (POC) with First In Human (FIH) implant and the launch of our iKnee Comfort Study.
The PFD/iOrthopedics Inc Resilient Arthroplasty Device (RAD), and its first product candidate the iKnee, has been IRB IDE authorized for human use in the first 10,000 qualified patients. Dr. Christopher Woodson is the Principal Investigator in the study. The device bridges the existing gap between non-invasive and totally invasive, ablative, barbaric, and antiquated surgery.
The update also shows the prototype post-production smoothing process has been approved. The prototype smoothing process occurs after the prototype is transferred from the 3D Printer and the exterior surface needs some additional refining. The smoothing process developed by AM Technologies, revolutionized specifically for RAD/iKnee, uses a green plant-based consumable solution on the iKnee Quadrathane PU material for optimum and exceptional results.

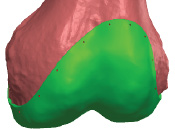

The images above show the iKnee prototype model with the perimeter borders being drawn out on the digitally prepared post sculptured processed knee of our first human candidate. The technology for the pre-surgical sculpturing was developed by Materialise and uses their Mimics software applied to CT manufacturers workstations. The images also show the iKnee initial drawings on the candidate’s femoral head and also the actual STL file image to be used for 3D printing.
PFD/iOI will market the iKnee orthopedic implant along with the iKnee Revenue program to hospitals, ASCs and payers, and will market a complete program based on superior outcomes, more economical treatment and pain reduction, and improved activities of daily living (ADL).
About NKC
Neuro-Kinesis Corporation (NKC) is an advanced medical device technology company created to consolidate and commercialize its leading-edge breakthroughs in surgical tools that integrate state-of-the-art biosensor technologies, advanced micro-miniaturization design and artificial intelligence to further the ability of a physician to treat their patients.
Interest in the NKC surgical tool technology has already been generated in several large medical device companies, and the company is on track to complete the continued prototyping and research studies required to bring their initial product candidates to pre-market commercialization.
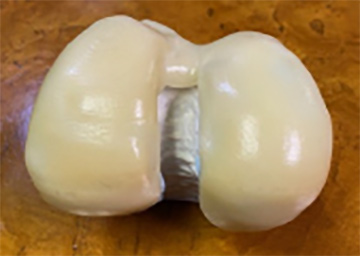
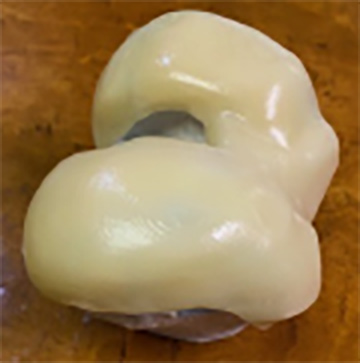
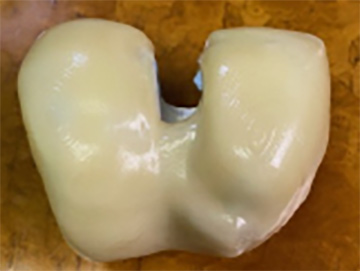
The images above show the smoothed iKnee surface created by the POST PRO smoothing process by AM Technologies.
About PFD/iOrthopedics
The PFD/iOrthopedics INC (PFD/iOI), Resilient Arthroplasty Device (RAD), and its first product candidate the iKnee, has been IRB IDE authorized for human use in the first 10,000 qualified patients. PFD/iOI is introducing a 21st Century, personalized, patient driven, novel and highly disruptive restorative joint salvage outpatient approach and effective solution to fill the gaping, costly, inefficient, and non-therapeutic void between pills, shots, bracings, physical therapies and limiting of daily activity for patients suffering from osteoarthritis and the much more dramatic, life altering and barbaric surgical replacement of joints with a simple outpatient procedure.

To learn more about the PFDMOF3001
CLICK HERE

To learn more about PFD/iOI
CLICK HERE
CONTACT
PFD Capital Partners
23161 Lake Center Dr. Ste. 100
Lake Forest, CA 92630
Email:
Office Phone:
(888) 475-4748
Fax Number:
(888) 475-4748


