about
PFD/iOrthopedics Inc. (PFD/iOI) is the developer of advanced implantable orthopedic devices designed to bring restorative function to patients suffering from a variety of orthopedic issues. Specifically to the PFDMOF3001 Opportunity Fund, LLC is their creation of a wholly new method for improving leg and knee function while reducing pain for those suffering from advanced arthritic disease or sports injury. Called the Resilient Arthroplasty Device (RAD), the technology utilizes a custom-made flexible implant that can cover the damaged end of a patient’s femur in order to restore movement without the need for bone removal, large metal implants, long recovery times, and the high cost associated with traditional Total Knee Arthroplasty procedures.
about
PFD/iOrthopedics Inc. (PFD/iOI) is the developer of advanced implantable orthopedic devices designed to bring restorative function to patients suffering from a variety of orthopedic issues. Specifically to the PFDMOF3001 Opportunity Fund, LLC is their creation of a wholly new method for improving leg and knee function while reducing pain for those suffering from advanced arthritic disease or sports injury. Called the Resilient Arthroplasty Device (RAD), the technology utilizes a custom-made flexible implant that can cover the damaged end of a patient’s femur in order to restore movement without the need for bone removal, large metal implants, long recovery times, and the high cost associated with traditional Total Knee Arthroplasty procedures.
background
PFD/iOrthopedics Inc. (PFD/iOI) was founded in 2017 to begin the process of bringing to market the innovative discoveries in orthopedic device technologies that Dr. Thomas Grotz had been developing over the past 30 years.
PFD/iOI has secured 25 patents both in the US and internationally covering several of these innovations, and with this strong IP portfolio, they stand in a secure position to achieve the regulatory requirements to bring their products to market. Specific work on the RAD technology and iKnee Resurfacing, which is the first product candidate using the RAD technology, began in 2012 when Dr. Grotz, one of San Francisco’s top orthopedic surgeons, became frustrated with the failures and the incomplete recoveries patients were having with the current Total Knee Arthroplasty (TKA) procedure. Dr. Grotz knew there had to be a better method and so he began work on a method that would be less invasive and had the ability to restore function while at the same time mitigating the long list of side-effects and failure rates that were associated with standard TKAs. He applied for the first patent on the RAD technology in 2014 and then fortified those claims with additional patents over the following year. Currently, PFD/iOI holds 16 patents on the technology which fully covers the unique differentiators that make the innovation a potential market disruptor in the orthopedic knee replacement sector.
vision
The Radial Arthroplasty Device (RAD) technology and the iKnee first product candidate are the results of the innovative vision of Dr. Thomas Grotz. Dr. Grotz is a noted orthopedic surgeon who has developed several novel inventions for advancing orthopedic care for a variety of patient issues. The RAD and the iKnee are just two of those inventions that were created to provide an orthopedic surgeon a better alternative than the current Total Knee Arthroplasty (TKA) procedure and traditional prosthetic hardware provides.
Currently, the TKA market represents a $50 billion financial burden for patients, insurance providers, and the healthcare system. And this number does not reflect the significant additional costs to the patient associated with lost productivity, post-surgical costs, lifetime restrictions, let alone potential complications from the procedure. PFD/iOI is focused on validating their iKnee Resurfacing through a comprehensive clinical trial and regulatory certification strategy in order to bring this procedure from trial to first application commercialization by the end of 2022. PFD/iOI is creating the protocols for its first clinical trial of up to 10,000 selected candidate patients for the iKnee Resurfacing procedure. The success of the first trial is expected to not only validate the procedure for further regulatory licensing but also to begin the longitudinal data gathering needed to prove the technology’s ability to not only provide immediate short-term pain relief but to confirm the iKnee Resurfacing’s ability to provide a long-term alternative to TKA.
As acceptance of the iKnee Resurfacing grows, iOi will be able to demonstrate to hospitals, ambulatory surgery centers, orthopedic surgeons, and insurance providers the superior outcomes, reduced financial cost, and greater patient care advantages that the RAD technology and the iKnee Resurfacing provide.
innovation
The PFD/iOI patented RAD technology and the iKnee Resurfacing, the first product candidate of the RAD innovation, presents a significant leap forward in providing restorative health to patients suffering from the effects of arthritis and trauma to the knee joint. In creating an option for select candidates who have not seen success with standard types of treatment, including physical therapy, steroid injections, or external mechanical support devices, and who are now facing a Total Knee Arthroplasty procedure, the RAD/iKnee combination provides a potentially superior option to recovery.
The RAD and the iKnee Technology
The patented RAD technology and the iKnee first product candidate provide orthopedic surgeons a potentially superior option in bringing restored health to their patients suffering from severe damage to the knee joint. By creating an arthroscopically implantable device that does not require the opening of the leg of a patient, the removal of large sections of bone, and the implantation of heavy hardware prosthetics, the RAD/iKnee allows a patient to be literally back on their feet in less time, at a lower cost, and with less chance of rejection or failure.
OVERVIEW
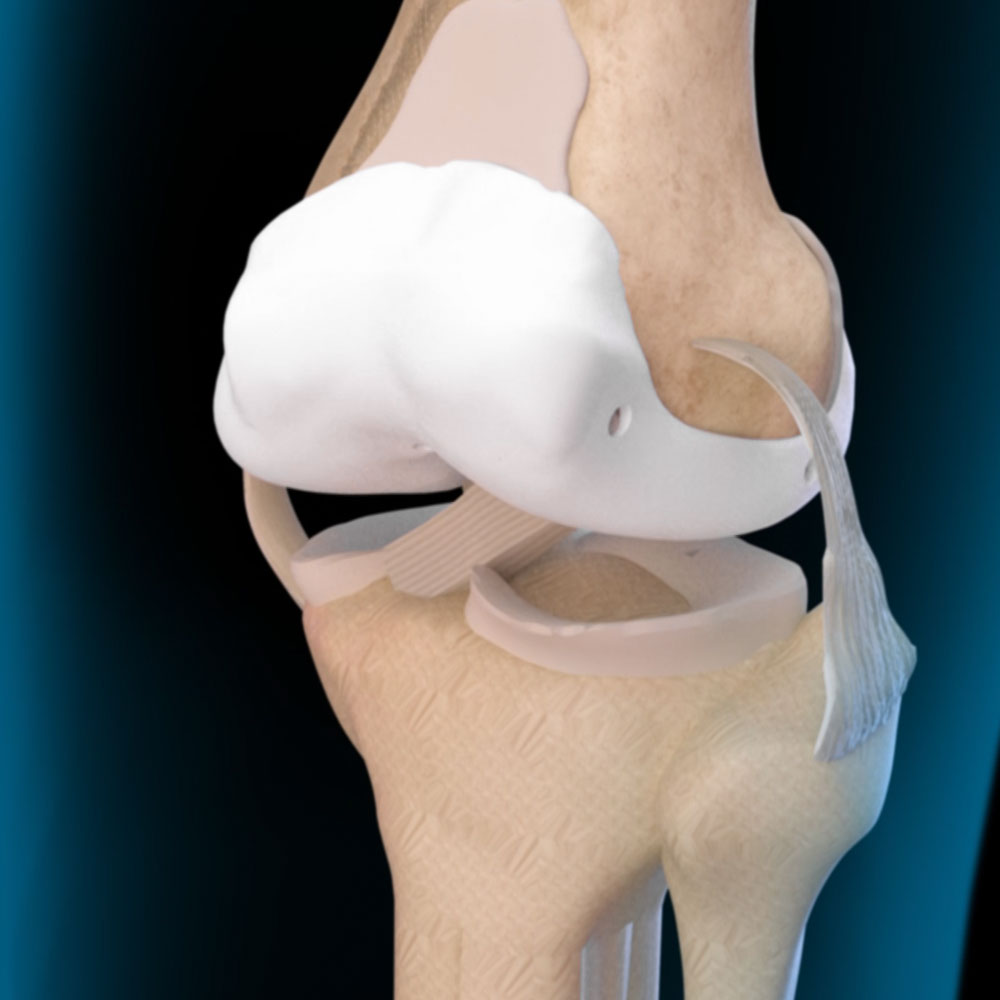
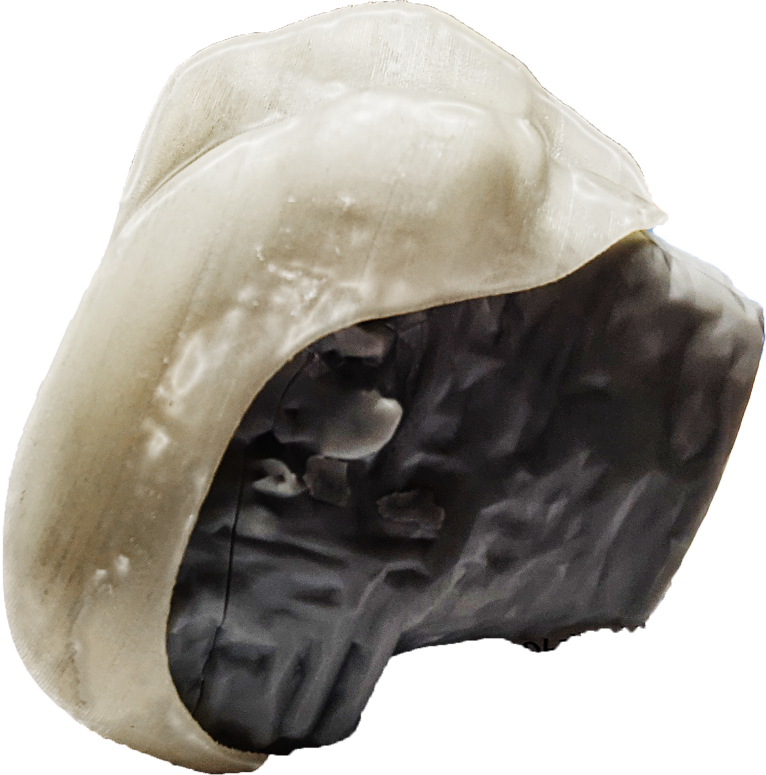
The patented Radial Arthroplasty Device (RAD) is a resilient femoral implant that acts as a cushion allowing for renewed joint motion. The RAD can endure all the variable joint forces and cyclic loads associated with normal activity and even heavy athletic motion while reducing pain and improving function after injury or disease. The iKnee Resurfacing represents the first product candidate of the RAD technology and it is now being advanced through the needed regulatory processes in order to allow human clinical trials to begin.
IRB CLINICAL STUDY
The Resilient Arthroplasty Device (RAD) initial application of the core technology iKnee seeks to provide temporary comfort and improved function for patients awaiting Total Knee Arthroplasty (TKA).
Reasons to delay a TKA include but are not limited to the need for weight loss, wound or infection healing, implant availability, and required medical or psychological patient preparations.
iKnee use determinations are made between the patient and caretaker.
The subject population is individuals with refractory disabling knee pain who failed other conservative measures and are on track for TKA.
The iKnee is expected to provide comfort for up to 6 months or as determined clinically. It is not intended to treat a disease, or underlying cause leading to TKA.
Objective: To study iKnee efficacy on quality of life and activities of daily living in patients awaiting TKA. The data will be used to improve possible designs to enhance patient comfort.
Procedure: A rolled medical polymer is inserted through a small parapatellar incision and then expanded to fit and pad the distal femur, providing a stable cushion made of medical polymers used safely and reliably for decades.
The procedure is expected to take about 30 minutes and involves an incision of 2-3-cm skin and sub subcutaneous tissue into the joint capsule, an incision less than one cm in depth. Then the iKnee device is inserted into the knee capsule.
No muscle, tendons, or ligaments are cut.
Study Rationale: Total Knee Arthroplasty (TKA) has become one of the leading operations for senior citizens, both in cost and frequency. When a patient has a disabling knee condition limiting mobility and quality of life there is only one medical option, TKA.
The TKA operation is a significant procedure, involving the removal of the bottom of the femur, top of the tibia, part of the patella to make space for the artificial metal and polyethylene prosthesis.
The iKnee as a temporary device, may significantly increase patient quality of life, functionality, and comfort while they await the knee replacement operation (TKA).
The iKnee procedure poses almost no medical risk to the patient, either as a procedure or as a risk to patient knee as the decision has already been made to remove the joint.
PFD/iOrthopedics (PFD/iOi) has made the Non-Significant Risk determination (NSR) paying close attention to all CFR’s and FDA Guidance documents.
INTRODUCTION
Patients who will be making application for inclusion within the study will read and execute the informed consent agreement and all related memorandums of understanding for the study before admittance to the study.
THIS IS THE FIRST STUDY IN WHICH THIS MEDICAL DEVICE INSERTION PROCEDURE IS BEING MADE AVAILABLE TO HUMANS.
PURPOSE OF THE STUDY
“Investigational” means the study medical device being tested has not been cleared by the US Food and Drug Administration.
The purpose of this study is to gather data to validate and improve a temporary procedure that will increase comfort and functionality of those awaiting a Total Knee Arthroplasty.
HOW LONG THE STUDY WILL LAST AND HOW MANY PEOPLE WILL BE IN THE STUDY
The iKnee as a temporary device will have different study time frames dependent on the patient and related physician(s). We expect a duration of one month minimum and 6 months maximum.
Data/reports from each participant will be collected and analyzed.
It is planned that up to 10,000 adult male and female study participants will be involved in the iKnee Comfort Study.
BENEFITS
The PFD/PFD/iOI RAD technology and the iKnee Resurfacing incorporate significant differentiators from any other product on the market, as well as providing a completely different, and potentially better option, for patients facing Total Knee Arthroplasty (TKA) surgery. Some of the benefits include:
- The technology has comprehensive IP protection through 16 US and International patents.
- The technology has already received Authorization for Human Use Allowance from the FDA.
- The technology has already received a Non-Significant Risk Device classification from the FDA allowing more rapid movement to first-in-man trials.
- Implantation of the iKnee is done on an outpatient basis via an arthroscopic procedure thereby reducing cost and recovery time.
- Each iKnee implant is custom-made to create a perfect fit for each patient to ensure maximum performance and reduce potential side effects associated with standard TKAs.
- In addition to the product technology, PFD/iOI is on a path to creating and developing a comprehensive Center of Orthopedic Excellence for surgeon training and demonstrations of the technology.
PFD/iOi and PFD
PFD Management has identified PFD/iOI ‘s RAD technology and its iKnee Resurfacing innovation as one of the five Medical Technology companies to include in the PFDMOF3001 Opportunity Fund, LLC. PFD Management believes that PFD/iOI is on track to meet a significant commercialization event within the fund’s time frame. To learn more about the specifics of this component of the fund, register for access at our Membership Portal.