OVERVIEW
cassderma Rx is the developer of an innovative prescription topical gel that supports the rapid regeneration of tissue for atrophic recalcitrant wounds. The gel is based on a patented formulation that uses a serum-free, nutrient-rich medium to the wound site along with a non-steroidal anabolic hormone to stimulate and support the growth of connective tissue originating from a wound bed and its peripheral walls. The gel has been found to be effective in reducing the healing time on a broad spectrum of wound types including diabetic ulcers, pressure ulcers, burns, and surgical wounds. The formulation has even been found to be effective in treating persistent long-term wounds where other current methods of regeneration have been unsuccessful.
The cassderma Rx Wound Healing Hydrogel represents a novel disruptive solution in the topical wound care market sector. The product’s patented formulation is the first to bring active nutrients in a cell culture medium along with a naturally occurring cell growth hormone that together is designed to not just protect a wound site while healing occurs but to promote and accelerate healthy cell and connective tissue repair in order for the cure to occur.
cassderma Rx is now working on finalizing its formulation to begin its POC animal wound study. This will be followed by a series of non-clinical toxicity trials needed in order to seek approval from the FDA for an Investigational New Drug (IND) authorization. Once approved, will begin its clinical studies for its development program (i.e., clinical, nonclinical, and CMC) after which the company will submit an NDA for market approval in order to move forward with its commercialization process.
WEBSITE: cassderma.com
EMAIL: info@cassderma.com
TEL:
ADDRESS:
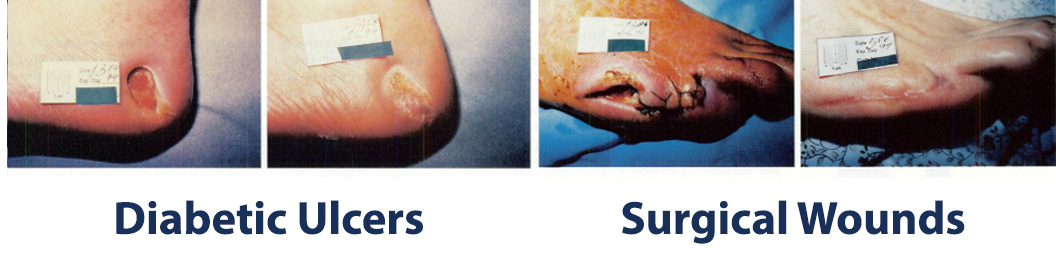
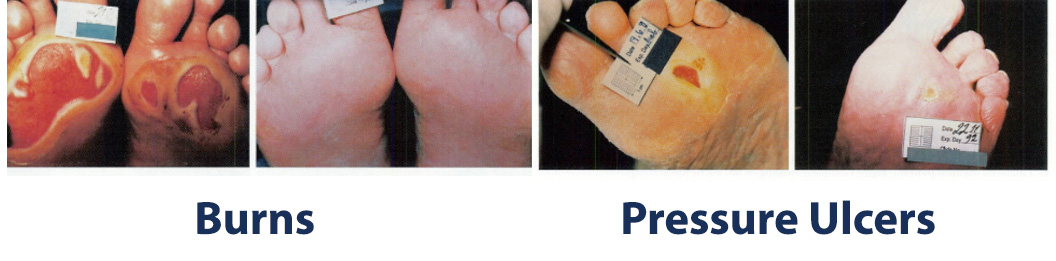
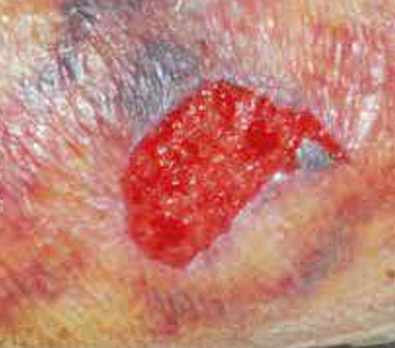
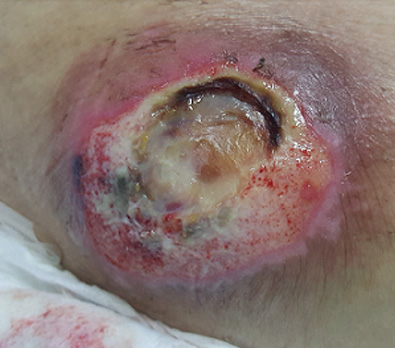
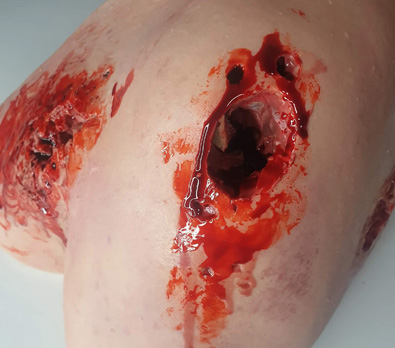
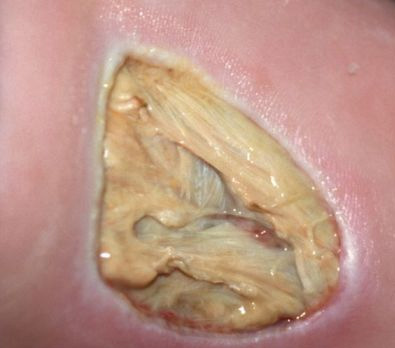
The images above show results from early studies done with CASS101 on a variety of wound types. cassdermaRx is seeking to replicate these results in their upcoming studies.
UNDERSTANDING CHRONIC WOUNDS
A chronic wound is defined as a wound that has been physiologically impaired due to a disruption of the wound healing cycle. This is usually because of impaired angiogenesis, innervation, or cellular migration, among other reasons. Epithelialization, or skin regeneration, varies depending on numerous factors, including comorbidities (eg, diabetes, autoimmune disease, peripheral artery disease), increased body mass index, anatomic location, and medications. Although there is no specific time frame that clearly differentiates an acute from a chronic wound, some suggest that the lack of approximately 15 percent reduction weekly or approximately 50 percent reduction of the surface area of the wound over a one-month period indicates a chronic state.
For such wounds, local treatment is directed toward reducing pain, minimizing infection and bleeding from the wound, and dealing with the most troublesome chronic wound problems that affect the patient physically and emotionally, Local care of chronic wounds includes debridement and proper wound dressings as well as preparing the wound bed to accept a skin graft or flap, or for closure, when possible.
The following shows the most common sources for chronic wound types:
Burns
Closed Surgical Wounds
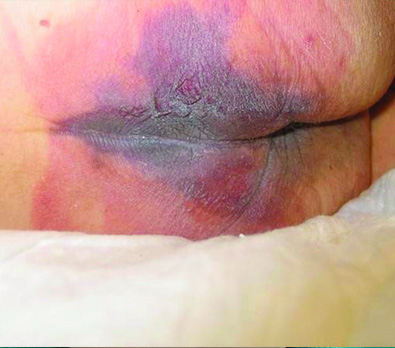
Deep Tissue Pressure Injury
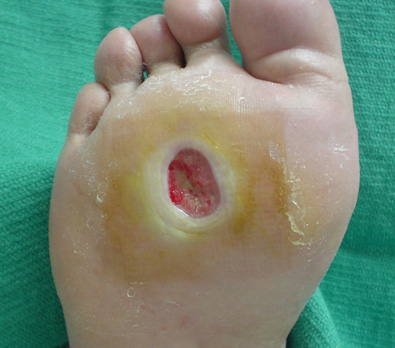
Diabetic Foot Ulcers
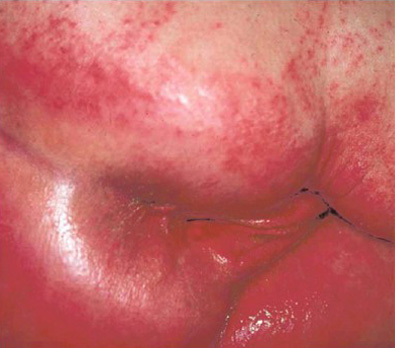
Incontinence Associated Dermatitis
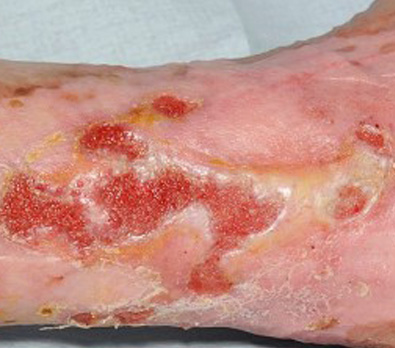
Leg Ulcers
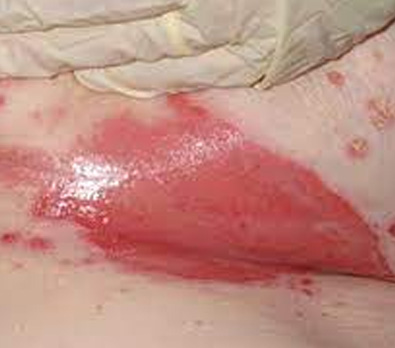
Moisture Associated Skin Damage
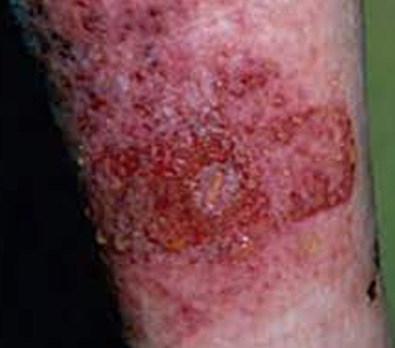
Medical Adhesive Related Skin Damage
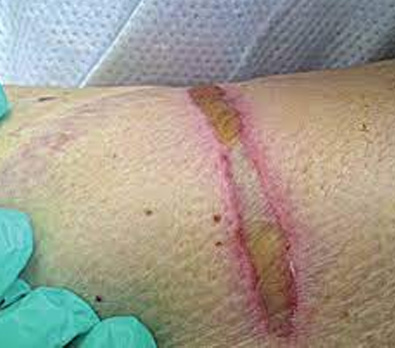
Medical Device Related Pressure Injuries
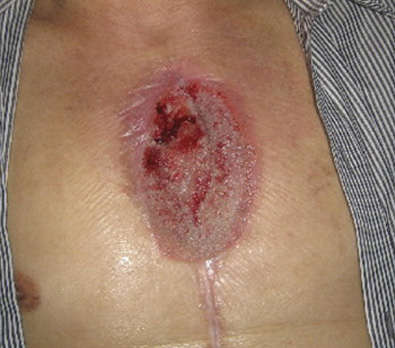
Open Surgical Wounds
Skin Tear
Stage 3 & 4 Pressure Injury
Trauma
Unstageable Pressure Injury
CURRENT WOUND TREATMENT OPTIONS
Successful treatment of difficult wounds requires assessment of the entire patient and not just the wound. Systemic problems often impair wound healing; conversely, non-healing wounds may herald systemic pathology. Regardless every wound goes through various stages of healing, depending on the type of wound and its severity. These stages are usually categorized into three categories:
Primary wound healing
Primary wound healing, or primary intention wound healing, refers to when doctors close a wound using staples, stitches, glues, or other forms of wound-closing processes.
Secondary wound healing
Secondary wound healing occurs when a wound that cannot be stitched causes a large amount of tissue loss. Doctors will leave the wound to heal naturally in these cases.
Tertiary wound healing
There are several types of wounds, depending on factors such as the source of the wound and any underlying issues that may lead to it. Wounds are typically open or closed. A closed wound is an injury that does not break the surface of the skin but causes damage to the underlying tissues. A bruise is a common example of this. On the other hand, open wounds break the surface of the skin and may also damage underlying tissues.
For open recalcitrant wounds, treatment is based on a combination of removing diseased or dead tissue, preventing infection, reducing inflammation and bleeding, and providing an environment for the tissue to regenerate naturally. To achieve this, physicians use a variety of items including:

Antimicrobial Dressing
Wound Cleansers

Compression Bandage
Wound Filler

Alginate Dressing

Foam Dressing
Hydrocolloid

Hydrofiber® Technology

Hydrogel Wound Dressing

Primary Dressing

Secondary Covering
Surgical Dressing
KEY HIGHLIGHTS
cassderma Rx was founded in 2020 to begin the process of bringing to market an innovative discovery in a curative wound healing topical product that has resulted from the decade-long work of Dr. Daniel Rastein. As an educator and noted industry leader in healthcare and biomedical research, Dr. Rastein has been involved in designing the regulatory standards of numerous drugs and biomedical devices for cutting-edge manufacturing, research, medical, aerospace, and agriculture companies.
The cassderma Rx Wound Healing Hydrogel is a first-of-its-kind patented solution for dealing with persistent chronic wound repair. The product and the company have several distinguishing differentiators including:
- The formulation utilizes a naturally occurring hormone for treatment of the wound with a nutrient-rich cell culture medium providing nutrition to the wound site.
- The combination of the hormone with a nutrient-rich cell culture medium, has been shown to be effective on a variety of chronic wounds, including deep burns, persistent sores, and diabetic ulcers.
- The company is ready to begin its POC animal wound model study followed by its non-clinical toxicity study in order to prepare for its IND approval.
- The awarding of the IND will result in a significant increase in the the valuation of the company with a 4x multiplier should a purchase exit option be taken.
- The company is headed by a carefully selected Executive Management Team who have the requisite skills across all clinical, toxicology, pharmacology, CMC, regulatory, and marketing fields to successfully bring a product of this type to a lead position in the pharmaceutical wound treatment marketplace.

INVESTMENT OVERVIEW
By packaging this startup equity investment within a proven revenue-generating program at PFD Management Opportunity Fund 3001, LLC (PFDMOF3001).
The investor receives:
- 5% annually on invested capital, distributed on a quarterly basis.
- 165% of invested capital back at the end of year 5 ($1.00 gets $1.65 at term)
The internal rate of return is 18% annually
- 5% annually for 5 years is 25%
- 165% at term is an additional 13% annually for 18% IRR
The investor has little else to be concerned with should the exit of this project take longer to exit, or the projected valuation varier from initial projections.
- Upon exit of this portfolio company, the fund will distribute the net proceeds to fund members proportional to their ownership in the fund.
Projected additional equity return:
- The targeted additional return is estimated for a 70% one-time distribution.
More then $10 billion is spent each year for wound care in the US.
Centers for Disease Control and Prevention (CDC). CDC Information on Infection Control
PRESENTATION
The cassderma Rx Wound Healing Hydrogel provides a new class of wound treatment that has the potential to bring faster healing to patients who suffer from a variety of wounds including diabetic ulcers, pressure ulcers, burns, and surgical wounds. For some patients who have not responded to current treatments, the cassderma Rx Wound Healing Hydrogel may offer an effective cure to healing long-term persistent wounds.
TAKE A LOOK AT THIS PRESENTATION TO SEE HOW AND WHY THE CASSDERMA RX WOUND HEALING HYDROGEL WILL MAKE AN IMPACT IN PROVIDING A BETTER OPTION FOR WOUND CARE.
PAIN POINT
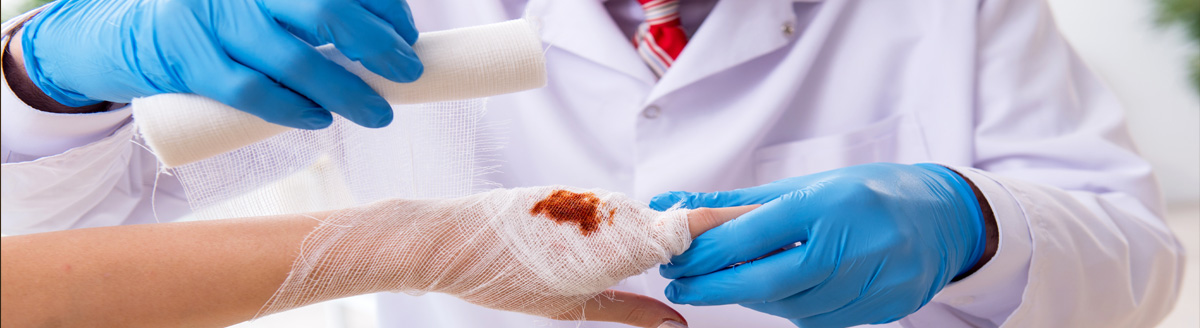
Wound Care Is A Global Pandemic
The National Council for Biotechnology Information, the World Health Organization, and the National Institutes of Health describe the need for wound care as a “worldwide pandemic” affecting 6.5 million patients in the US alone costing over $25 billion annually and rapidly growing due to increase in healthcare costs, aging population and the sharp rise in obesity and diabetes
-
1000 patients in hospitals die each week from pressure ulcers.
-
84% of amputations for diabetes patients are attributed to open wounds.
-
A 2000 study showed that 40 million inpatient surgical procedures and 31.5 million outpatient surgeries were performed in the US. All of these patients required some level of wound care post-operatively.
WOUND (noun) /wo͞ond/: a break in the continuity of the skin, caused by a traumatic injury such as burn, cut, or scrape, exposure to excessively hot or cold temperatures, chemical damage due to acid or alkaline burns or damage by radiation.
TREATMENT: application of various antibiotics and bandages or alternatively, the wound is closed by surgical procedures of suturing the wound’s edges, by grafting cultured skin, or by split skin grafts
PURPOSE: avoid complications such as infection and inflammation and promote the growth of granulation tissue that may or may not follow promptly, depending on adequate blood supply bringing into the wound the nutrients that the cells need for growth.
COMPLICATIONS: wounds can become infected, the blood supply can be insufficient for tissue growth, or contamination overcomes the antiseptics. In these cases, the healing process fails, and the wound becomes chronic. This is the case in most hospitals with pressure ulcers, unsanitary conditions, or underlying conditions, such as diabetes, venous insufficiency, or compromised immune system.
Nothing exists in the world to make wounds heal faster on their own than our body’s own healing power.
cassderma Rx is utilizing that power for the first time to bring a powerful solution for Chronic Wound Repair.
20 Million people need wound care treatment in the U.S. each year.
Research and Markets. Industrial and Institutional Cleaning Products – Global Market Trajectory 14 & Analytics Report, July 2020.
ELEVATOR PITCH
- Wound care is becoming a growing pandemic around the globe.
- With the aging population, increase in diabetes, obesity, and invasive surgical operations, the need for better wound treatment is critical for those whose wounds are persistent or do not respond well to traditional treatments.
- cassderma Rx has created a patented prescription topical gel that has been shown to provide relief and healing to patients with persistent wound care needs.
- The formulation utilizes a serum-free, nutrient-rich cell culture media supplement, along with a naturally occurring hormone that supplies the cells in the wound with the nutrients and the cell-growth stimulation required for the regeneration of tissues to treat recalcitrant chronic wounds.
- cassderma Rx is working to complete its formulation to begin its initial pre-clinical toxicity trials to show its proof-of-concept.
- The pre-clinical trials will gain the data needed to proceed with securing the regulatory approvals needed by the FDA for its IND designation.

ADDRESSING THE MARKET
The market for both procedures and materials needed for treating wound care represents a $20B a year sector affecting more than 20 million people in the U.S. alone. Material cost for items such as dressings, sutures, antibiotics, salves, and other topical and oral items is a major part of that market sector. For patients with persistent wound care needs, the cost for basic dressing supplies can reach $1,300.00 annually alone 1
Wound management has made rapid advances over the last 25 years with the increase in the understanding of the biology of chronic non-healing wounds. There are many product solutions in the wound care topical ointment and gel category. All of these fall into either an antiseptic or antimicrobials framework. Treatment selection depends on the practitioner’s skilled assessment of the wound and his knowledge of how to provide the optimum wound healing environment through the use of modern interactive dressings.
The cassderma Rx Wound Healing Hydrogel is uniquely positioned as it is the first product of its kind in the topical wound care category that has an ability not to just clean and protect against infection while the body heals itself, but rather to actually accelerate the body’s own regeneration process by providing wound-site delivery of nutrients along with a naturally occurring cell-growth hormone. Though effective for all types of wound care, cassderma Rx‘s primary focus is on providing a solution for physicians who need a solution for treating those patients whose wounds are persistent and recalcitrant. In this sector of the market, there are a limited number of effective topical options that are utilized currently, but none of these promote actual cell growth of the connective tissues in order to effect a cure.
As the cassderma Rx Wound Healing Hydrogel product is targeted for treating a chronic wound, it is a prescription topical gel formulation and will require a physician’s prescription for use. Because of this, the product will have a higher price point which will be covered by insurance reimbursement. Targeting this market sector, even with minimal share achievement, will mean a substantial revenue stream for the producing company.
As the market has shown, effective topical cure products have garnered significant value in their acquisition. Examples of this include Eli Lilly’s purchase of Dermira ‘s eczema cream for $1.3 billion, and Nestlé’s Skin Health Division’s purchase of Galderma, a dermatological skin-care product developer, for $10.5 billion in 2019.
The management of cassderma Rx even at its early stage of development has been approached by large players in this space interested in the product’s differentiators and potential.
1. https://www.woundsource.com/blog/purchasing-wound-care-supplies-potential-patient-hurdles#:~:text=Steps%20for%20Helping%20Patients%20Acquire%20Wound%20Care%20Supplies&text=Most%20wounds%20do%20not%20heal,annually%20for%20basic%20dressing%20supplies.
6.7 million people suffer from non-healing advanced wounds.
INTELLECTUAL PROPERTY
PATENTS
cassderma Rx has filed and been granted a patent with multiple allowances on the formulation and methods for stimulating wound healing. The patent thoroughly describes the unique differentiators that make the cassderma Rx Wound Healing Hydrogel a first-of-its-kind product.
PROOF OF CONCEPT
cassderma Rx is now working on finalizing its formulation to begin the initial pre-clinical toxicity trials needed for establishing its proof of concept. Based on the outcome of the trials, the company will seek FDA approval for an Investigational New Drug (IND) authorization to move forward with its next phase of clinical trials towards its commercialization process.
MANAGEMENT
cassderma Rx has been able to secure a top C-Level team to steer the company to its stated goals. The team’s broad areas of expertise and decades of experience in taking companies from startups to lucrative market positions are well placed for bringing cassderma Rx to either a high valuation for a purchase exit strategy or to make it ready for commercialization into the marketplace.
SCALABLE BUSINESS MODEL
As cassderma Rx’s Wound Healing Hydrogel presents a potential market disruptor in its market sector, it has several advantages to scalable growth as well as some challenges. cassderma Rx’s strategy for growth is positioned for two potential outcomes. One is to pursue its FDA regulatory process for an Investigational New Drug (IND) number. Achieving this will result in a significantly higher valuation for the company, which is anticipated to be at least a 4x multiplier. This new valuation would be able to position the company for a purchase exit from a key player in the industry. The second option is to continue its path to an NDA at which point it will be able to move forward with its own commercialization strategy. Should even a 1% market share be realized in the first year of commercialization, this could represent $100M in sales.
With either end goal, cassderma Rx is moving forward to finalizing the needed requirements for FDA approval. This strategy involves:
-
- Development of the Product Formulation and the Chemistry Manufacturing, and Control (CMC) requirements including:
- Selection of components (excipients) that provide for a stable topical gel formulation.
- Optimizing maximum levels of hormone and cell culture medium within the gel formulation without compromise to target product viscosity that is important in maintaining product residence within the wound site.
- Scale-up and refinement to GLP drug manufacture requirements for preclinical efficacy and toxicology studies to include:
- A 14-Day Hypersensitivity Study in Guinea Pigs
- A 3-Day Ocular Safety Study in Rabbits
- A 3-Month Dermal Toxicity Study in Mini-pigs
- Development of the Product Formulation and the Chemistry Manufacturing, and Control (CMC) requirements including:
Projected delivery is six months notwithstanding risk issues such as legal, bandwidth, further pandemic barriers
- An FDA Pre-IND Meeting to include:
- Advisement on data to support an IND application.
- Discussion on First in Human (FIH) clinical study.
After IND submission, cassderma Rx will determine its decision to pursue a purchase exit or continue onto the next phase of product development.

COMPETITIVE LANDSCAPE
Although there are many competitors in the topical ointment and gel wound care market sector, none of these are able to make a claim or show an ability to provide an effective method of promoting cell growth to enhance and accelerate the healing of wounds. cassderma Rx feels its unique formulation sets it in a category of its own and is well placed to be a disruptive product that will be able to dominate its market position.
BEPANTHEN® SENSIDERM CREAM
PuraPly AM

GrafixPL Lyopreserved Placental Membrane

Vericel Epicel
Vericel NexoBrid

Merz Contractubex

SKILLED LEADERSHIP TEAM
cassderma Rx brings together a skilled Executive Management Team whose broad range of experience provides the company with the leadership and know-how to be able to grow the company to meet its stated goals. Individuals on the team have proven track records of success in pharmaceutical formulations, wound care treatment, FDA and International regulatory procurement, IP protection, sales and marketing, and medical industry business growth and management. The company has set a well-considered and attainable path for the needed R&D, clinical trial data gathering, and regulatory securitization in order to position the company for either a high-valuation purchase exit or the ability to commercialize and enter the market on its own.
EXECUTIVE MANAGEMENT TEAM

Daniel Rastien, MD, MPH
Co-founder and CEO

Mike Sweeting
Vice-President of Regulatory

Partner & President, Pharmaceutical R&D/Regulatory Affairs for Global Markets, IAA Consulting LLC

Monil Shah, PharmD, MBA
Vice-President of Clinical Development

K&L Gates
Legal Consultants
HISTORY OF EXCELLENCE
The members of the cassderma Rx Executive Management have extensive backgrounds in each of their respected fields. Their expertise and abilities are highly sought after by top global companies and have contributed significantly to their respective successes.




RISK FACTORS
The following is not an exhaustive list of risk factors that may impact the PFDMOF3001 and or this particular portfolio company’s ability to fulfill its goals and/or negatively impact stakeholder value.
In evaluating the PFDMOF3001, the Company itself, and the portfolio company described within these pages and its business, one should carefully consider the following risk factors.
Challenges of penetrating the market with new technology:
Businesses, which are often under pressure to cut costs, are often hesitant at adopting new technologies. There is a chance that cassderma Rx will have a difficult time selling large quantities of its product.
Market Messaging:
Though the cassderma Rx Wound Healing Hydrogel is a first-of-its-kind product in the topical ointment and gel wound care product sector, other products already exist. This fact may prove challenging from a marketing perspective. If cassderma Rx is unable to deliver a clear message to its potential customers of the benefits of its system (accuracy, time, etc.) it will have a hard time selling its product.
Competition:
If another company succeeds in bypassing cassderma Rx‘s barriers to entry (patents, technological research, and know-how), cassderma Rx may be required to lower its price to stay competitive, this will cause the company’s margins to shrink and will hurt its bottom line.
Venture Capital Speculation:
Venture capital is highly speculative and entails significant risk. Due to this, investments should not be made by investors who cannot afford to lose their entire investment. In addition, these types of securities are illiquid, and investors cannot predict when to expect a return on their investments, if any. Even an exit event for a company may only yield a partial, if any, return on investment.
Liquidity:
Investments made via our fund are generally illiquid. This means that once you have committed your money it could be difficult for you to exit your investment and get your money back at a time that suits you. There is no secondary market for your investments.
Control:
By making an investment in PFDMOF3001 fund, you acknowledge that you are making a long-term investment. You will not have control over the day-to-day decisions made in relation to a particular investment or the timing of your exit.
Tax Issues:
You must ensure that you are aware of your tax obligations or any related risks that might apply to you as a result of any investment made by you in this fund. We encourage you to consult with appropriately qualified tax professionals regarding your tax circumstances.
Financial Risks:
Companies which are financed through venture capital may not be fully capitalized. The company may need to raise additional significant funds in the future in order to realize its business plan, which may dilute an investor’s holdings. There is no guarantee that the company will be able to secure future funds.
Execution Risk:
A company may be unsuccessful in executing its business plan for a variety of unforeseen factors. Business plans are necessarily based on a series of assumptions, some of which may not materialize as thought. Such factors include but are not limited to unforeseen challenges in research & development, unforeseen delays in securing key partnerships such as manufacturing, distribution, and marketing partners as well as delays in securing sales.
Adverse Economic Conditions:
Unfavorable changes in economic conditions, including inflation, recession, foreign currency fluctuations, political instability, or other changes in economic conditions, may have an impact on the overall investment environment for early-stage venture investments.
Management Risks:
Early-stage companies depend on a small key management team to make critical corporate decisions. Such a team may prove to be unreliable or ill-equipped for long-term corporate leadership or may simply leave for other opportunities.
Technological Risks & Defensibility:
The company’s underlying technology or intellectual property could be rendered obsolete, ineffective, or invalid by competitors or new technological breakthroughs.
Market Risks:
Even profitable companies are vulnerable to numerous external risks such as fluctuations in market trends, which could negatively impact a company’s core technology or assets, especially those companies which target a narrow sector of the market.
Regulatory & Legal Risks:
Changing regulatory and legal environments may have a significant impact on early-stage companies, complicating or outlawing key success factors. Additionally, your investment is not covered by the US FDIC or UK’s FSCS or other regulatory arrangements, nor any other statutory or voluntary compensation scheme.
Geo-Political Risks:
Early-stage companies that operate in unstable geopolitical regions may be vulnerable to events such as civil unrest, war, and sanctions.
DISCLAIMER
FORWARD-LOOKING STATEMENTS
CERTAIN INFORMATION SET FORTH IN THIS SUMMARY CONTAINS “FORWARD-LOOKING INFORMATION” UNDER APPLICABLE SECURITIES LAWS (“FORWARD-LOOKING STATEMENTS”). EXCEPT FOR STATEMENTS OF HISTORICAL FACT, INFORMATION CONTAINED HEREIN CONSTITUTES FORWARD-LOOKING STATEMENTS AND INCLUDES,
BUT IS NOT LIMITED TO, THE (I) PROJECTED PERFORMANCE OF THE COMPANY; (II) COMPLETION OF, AND THE USE OF PROCEEDS FROM, THIS FINANCING; (III) THE EXPECTED DEVELOPMENT OF THE COMPANY’S BUSINESS, PROJECTS, AND PARTNERSHIPS; (IV) EXECUTION OF THE COMPANY’S VISION AND GROWTH STRATEGY, INCLUDING WITH RESPECT TO FUTURE MARKETPLACE ADOPTION, PARTNERSHIP ACTIVITY, AND MARKET GROWTH; (V) SOURCES AND AVAILABILITY OF THIRD-PARTY FINANCING FOR THE COMPANY’S OPERATIONS; (VI) COMPLETION OF THE COMPANY’S ACTIVITIES THAT ARE CURRENTLY UNDERWAY, IN DEVELOPMENT OR OTHERWISE UNDER CONSIDERATION; (VI) RENEWAL OF THE COMPANY’S CURRENT CUSTOMER, MEMBER, PARTNERSHIP AND OTHER MATERIAL AGREEMENTS; AND (VII) FUTURE LIQUIDITY, WORKING CAPITAL, AND CAPITAL REQUIREMENTS. FORWARD-LOOKING STATEMENTS ARE PROVIDED TO ALLOW POTENTIAL INVESTORS THE OPPORTUNITY TO UNDERSTAND PFD’S BELIEFS AND OPINIONS BASED ON THE COMPANY’S BELIEFS AND OPINIONS WITH RESPECT TO THE FUTURE. THESE STATEMENTS ARE NOT GUARANTEES OF FUTURE PERFORMANCE AND SHOULD NOT BE RELIED ON IN ANY WAY. SUCH FORWARD-LOOKING STATEMENTS NECESSARILY INVOLVE KNOWN AND UNKNOWN RISKS AND UNCERTAINTIES, WHICH MAY CAUSE ACTUAL PERFORMANCE AND FINANCIAL RESULTS IN FUTURE PERIODS TO DIFFER MATERIALLY FROM ANY PROJECTIONS OF FUTURE PERFORMANCE OR RESULT EXPRESSED OR IMPLIED BY SUCH FORWARD-LOOKING STATEMENTS. ALTHOUGH FORWARD-LOOKING STATEMENTS CONTAINED IN THIS PRESENTATION ARE BASED UPON WHAT PFD AND THE MANAGEMENT OF THE COMPANY BELIEVE ARE REASONABLE ASSUMPTIONS, THERE CAN BE NO ASSURANCE THAT FORWARD-LOOKING STATEMENTS WILL PROVE TO BE ACCURATE, AS ACTUAL RESULTS AND FUTURE EVENTS COULD DIFFER MATERIALLY FROM THOSE ANTICIPATED IN SUCH STATEMENTS.
SUMMARY of TERMS
provides the seed capital of $500,000 for costs associated with the delivery of agreed-upon initiatives of the preferred stock purchase agreement
to meet the projected needs to achieve the target goals listed below.
+
Invested capital is evidenced through a
convertible preferred stock purchase agreement with 200% MOIC
required to convert to 5% common equity of cassderma Rx
+
Future rights at
20%
discount to fund future rounds
=

has targeted this portfolio company to provide
an additional 70% distribution to members upon exit
Target Goals
Current Round
-
(CMC) Chemistry Manufacturing Composition
-
Development of the Product Formulation.
-
Perfecting the Cell Culture Medium (CCM)
-
Developing the Delivery Method
Package A
-
Preclinical efficacy & toxicology animal study
-
Projected delivery, during this round (6 months)
-
Subject to the standard risk of time, legal, bandwidth, and future impact of pandemic.
Package B
-
FDA Pre-IND Meeting.
-
Process of application and support submission.
-
Purpose to seek and receive an Investigational New Drug (IND) status.
Once Achieved the Commercial Potential of the Company will be assessed with the hopes that:
-
The product achieves a significant valuation and becomes highly desirable at this stage.
-
There is significant potential for an exit.
SPONSOR
PFD Capital Partners
Lake Forest, CA 92630
Email:
info@PFDCap.com
Office Phone:
(888) 475-4748
VENDOR
PFD Management LLC
Lake Forest, CA 92630
Email:
team@pfdmanagement.com
Office Phone:
(888) 475-4748
-
The cassderma Rx program is not intended to become an operating company. The founders intend to develop the technology for sale to a large international medical products leader.
-
It is anticipated that the sale of the technology will be a minimum of $200,000,000.
-
When this occurs, the members of the PFDMOF3001 would receive $4,975,000. Which is an additional 20% of the fund’s total capital of $25,000,000.00.






